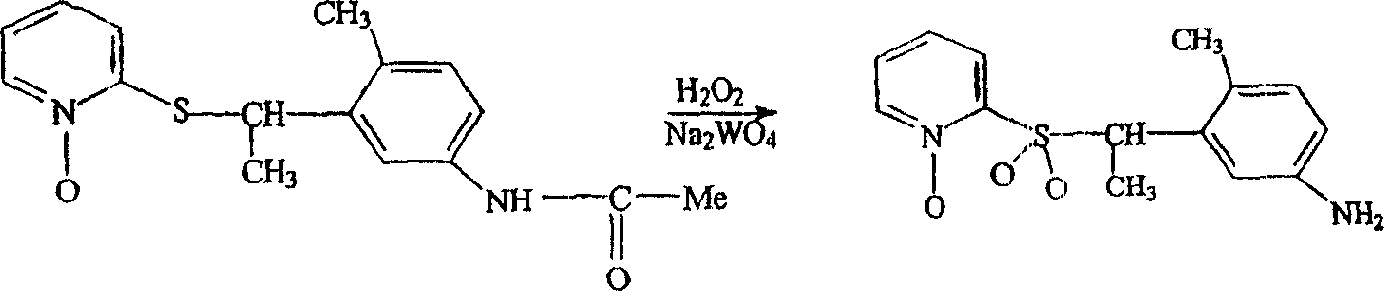Antiretroviral pyridine and quinoline derivatives
A technology of pyridine and sulfonylpyridine, applied in the field of pyridine and quinoline derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of 2-[[1-(5-amino-2-methylphenyl)ethyl]sulfonyl]-pyridine-N-oxide
[0045]
[0046] (compound 1)
[0047] Add 0.12 g Na 2 WO 4 , followed by the addition of 35% hydrogen peroxide over 20 minutes. The exothermic reaction was cooled using a room temperature water bath. The mixture was stirred for 20 min, then an additional 1.3 g of 35% H 2 o 2 . The mixture was allowed to warm to 35-43°C for 2 hours, then stirred at room temperature overnight. The mixture was then treated with 5 ml ethanol, 10 ml water and 5 ml cone. HCl and heated on a steam cone for 1 hour. The mixture was cooled, diluted with 20ml of water and filtered to remove solids. The filtrate was made basic with concentrated aqueous ammonium hydroxide to give a sticky solid. The solid was dissolved in 30 ml of water and 3 ml of cone. HCl and some insoluble material was filtered off. The filtrate was basified and a solid precipitated which was filtered off, washed with water and dried ov...
Embodiment 2
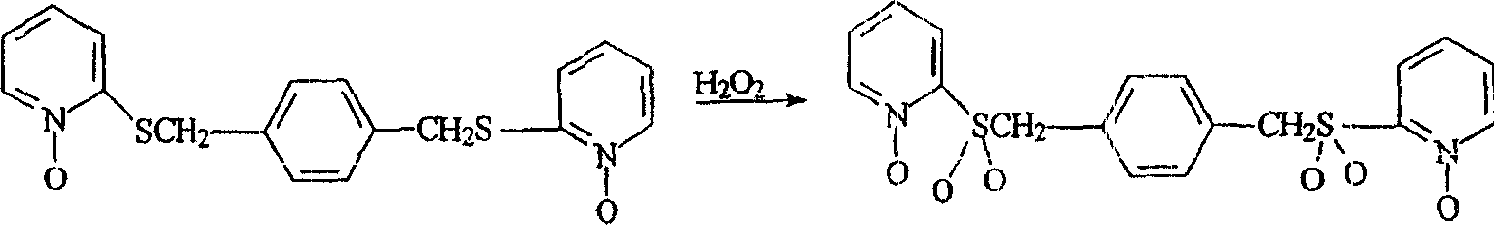
[0049] Preparation of 1,4-xylyl-bis-2-sulfonylpyridine-N-oxide
[0050]
[0051] (compound 23)
[0052] To a mixture of 14 g 1,4-xylyl-bis-2-thio-pyridine-N-oxide in 175 ml glacial acetic acid was added 20 ml H 2 o 2 (30% aqueous solution). The mixture was stirred over the weekend before adding another 8 ml of 30% H 2 o 2 , and the mixture was heated to 50-60 °C for 2 hours. The mixture was cooled and evaporated to dryness. Chloroform was then added and the mixture was boiled, then cooled and left overnight. The insoluble product was filtered off, washed with ethanol and then with chloroform. 13.6 g of final product were obtained, melting point 233-235°C. C 18 h 16 N 2 S 2 Anal. Calcd. for: C = 51.42; H = 3.84; N = 6.66. Found values: C=47.39, H=4.10; N=6.74.
Embodiment 3
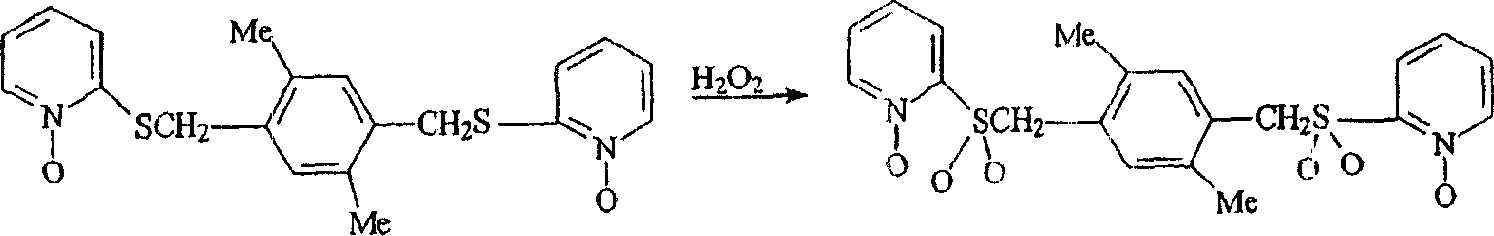
[0054] Preparation of 1,4-[1,2,4,5-tetramethylbenzyl]-bis-(2'-sulfonylpyridine-N-oxide)
[0055]
[0056] (compound 25)
[0057] To a mixture of 1,4-[1,2,4,5-tetramethylbenzyl]-bis-(2'-thiopyridine-N-oxide) in 175 ml of glacial acetic acid was added 25 ml of 30% H 2 o 2 aqueous solution. The reaction mixture was stirred overnight, then an additional 25 ml of 30% H 2 o 2 aqueous solution, the mixture was heated at 50-60°C for 4 hours. Then 600ml of water and 100g of ice were added. The white solid was filtered off and dried at room temperature, melting point 242-244°C. The recovery rate was 2.2 g. Infrared spectra were consistent with this structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com