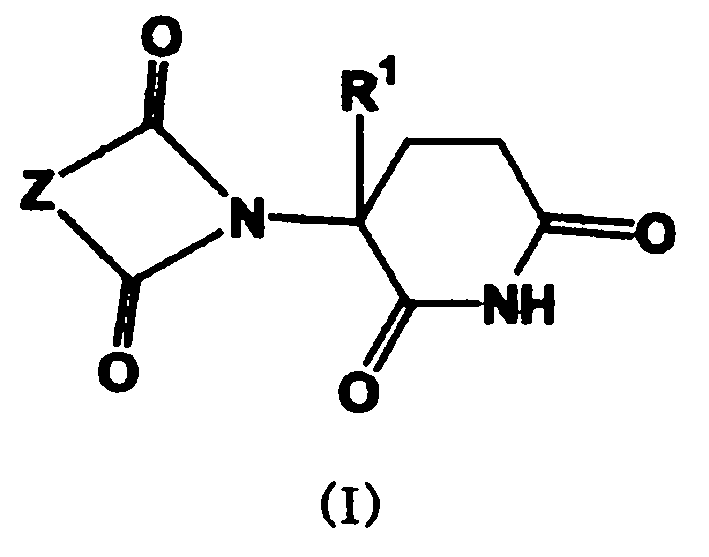
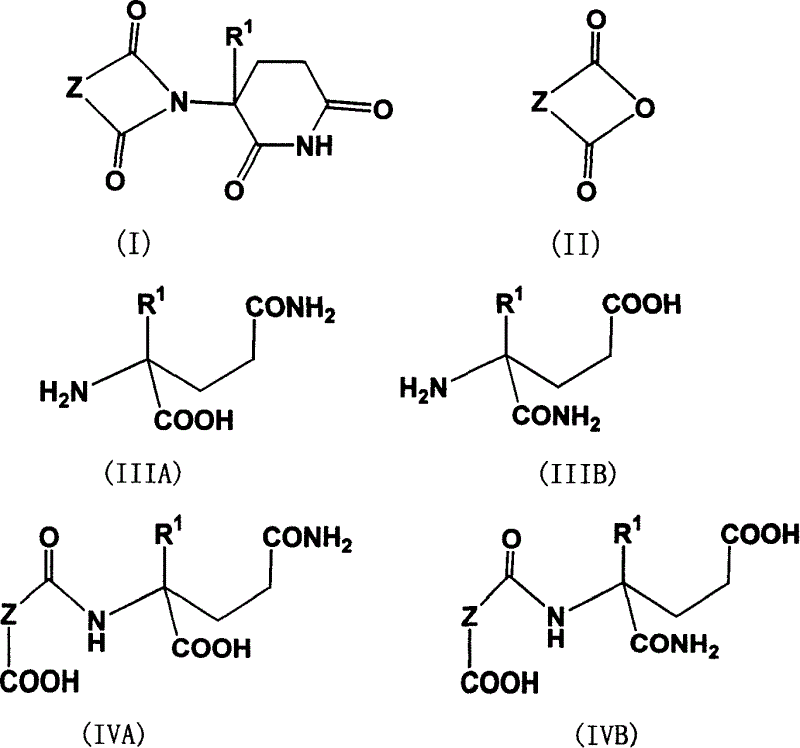
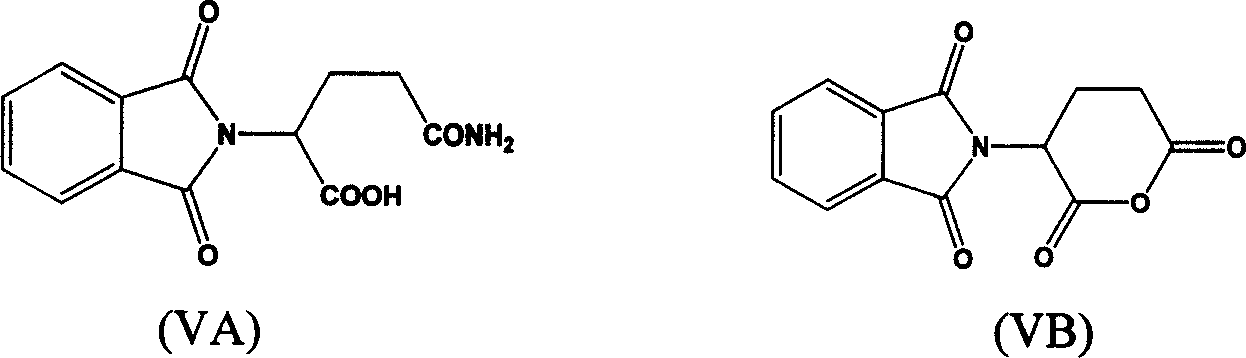
New method of synthesizing thalidomide and its derivative
A compound and mixture technology, applied in the field of preparing phthalamide piperidone and derivatives thereof, can solve the problems of difficult post-processing purification, long reaction steps, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 3-(1,3-Dihydroisoindol-1,3-dione-2-yl)piperidine-2,6-dione(phthalamidinone)phthalic anhydride (1.48 g, 10 mmole ) and glutamine (1.40 g, 9.5 mmole) in the mixture of water (5ml) and triethylamine (5ml), stirred at room temperature for 6 hours, and evaporated under reduced pressure to remove water and triethylamine to obtain (IIIA) (R 1 H, Z is a triethylamine salt of a benzene ring), the latter and carbonyldiimidazole (3.56 grams, 20 mmole) were refluxed in dry THF (15 ml) for 14 hours, cooled to room temperature and filtered, and the filter cake was washed with THF ( 10 mL) and dried in vacuo overnight to obtain 1.82 g of a white solid with a yield of 76%. M.p. 269°C-272°C. 1 H NMR (CDCl 3 , ppm) δ8.05 (br, 1H), 7.88-7.90 (m, 2H), 7.76-7.79 (m, 2H), 4.97-5.03 (m, 1H), 2.72-2.95 (m, 3H), 2.14- 2.20 (m, 2H).
Embodiment 2
[0032] 3-(1,3-Dihydroisoindol-1,3-dione-2-yl)piperidine-2,6-dione (phthalamidinone)
[0033] The reaction procedure is the same as that in Example 1. In the first step, dimethylformamide is used instead of water to obtain 1.26 g of white solid with a yield of 49%. M.p. 269°C-272°C.
Embodiment 3
[0035] 3-(1,3-Dihydroisoindol-1,3-dione-2-yl)piperidine-2,6-dione (phthalamidinone)
[0036] The reaction procedure was the same as that in Example 1. In the first step, dimethylformamide was used instead of THF to obtain 1.36 g of a white solid with a yield of 53%. M.p.268°C-270°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com