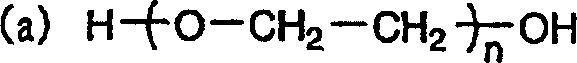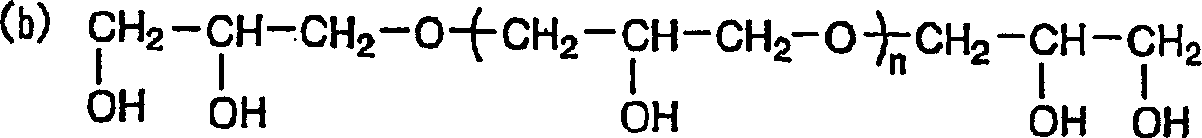Electrochemical cell
An electrochemical and battery technology, applied in the field of electrochemical batteries, can solve the problems of rapid charge/discharge characteristics, degradation of cycle life characteristics, large interface resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
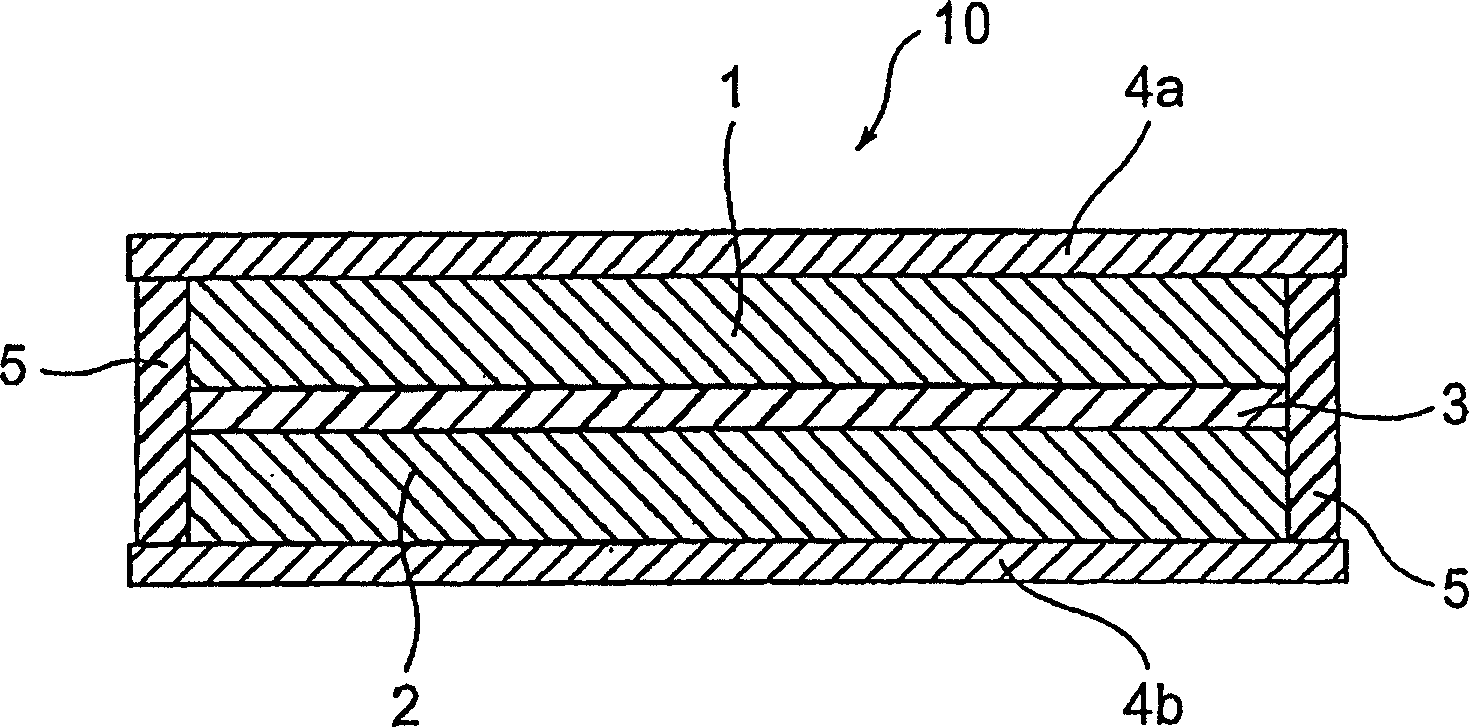
[0054] By adding 72% by weight of 5-cyanindole trimer represented by the following chemical formula as the positive electrode active material, 20% by weight of vapor-phase grown carbon as a conductive aid, and 8% by weight of polyvinylidene fluoride as a binder (average molecular weight: 1100) were combined, the mixture was stirred and mixed by a stirrer, and the mixture was molded into a given size by hot pressing to prepare a positive electrode layer 1 .
[0055]
[0056] By combining 75% by weight of polyphenylquinoxaline represented by the following chemical formula as a negative electrode active material and 25% by weight of vapor-phase grown carbon as a conductive aid, stirring and mixing the mixture by a stirrer, and passing the mixture by hot pressing Molded to a given size, the negative electrode layer 2 was prepared.
[0057]
[0058] The positive electrode layer and the negative electrode layer 1 and 2 impregnated with the electrolyte are oppositely arranged t...
Embodiment 2
[0067] An electrochemical cell was prepared in the same manner as in Example 1, except that 0.5% by weight of polyethylene glycol with an average molecular weight of 4,000 was added to the electrolytic solution as an electron transfer accelerator. The measurement results of its characteristics are shown in Table 1. It can be seen from Table 1 that compared with the electrochemical cell of Comparative Example 1, the capacity and cycle life of the electrochemical cell of Example 2 are increased by 7% and 25% respectively.
[0068] After charging under constant current / voltage (CCCV) 5C, 1.2V, 10 minutes and discharging to 0.8V at constant current (CC) at 20C, the battery was compared with the battery of Comparative Example 1 charged / discharged under the same conditions Compared with the residual capacity increased by 50%.
Embodiment 3
[0070] An electrochemical cell was prepared in the same manner as in Example 1, except that 0.5% by weight of polyethylene glycol with an average molecular weight of 20,000 was added to the electrolytic solution as an electron transfer promoter. The measurement results of its characteristics are shown in Table 1. It can be seen from Table 1 that compared with the electrochemical cell of Comparative Example 1, the capacity and cycle life of the electrochemical cell of Example 3 are increased by 8% and 20% respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com