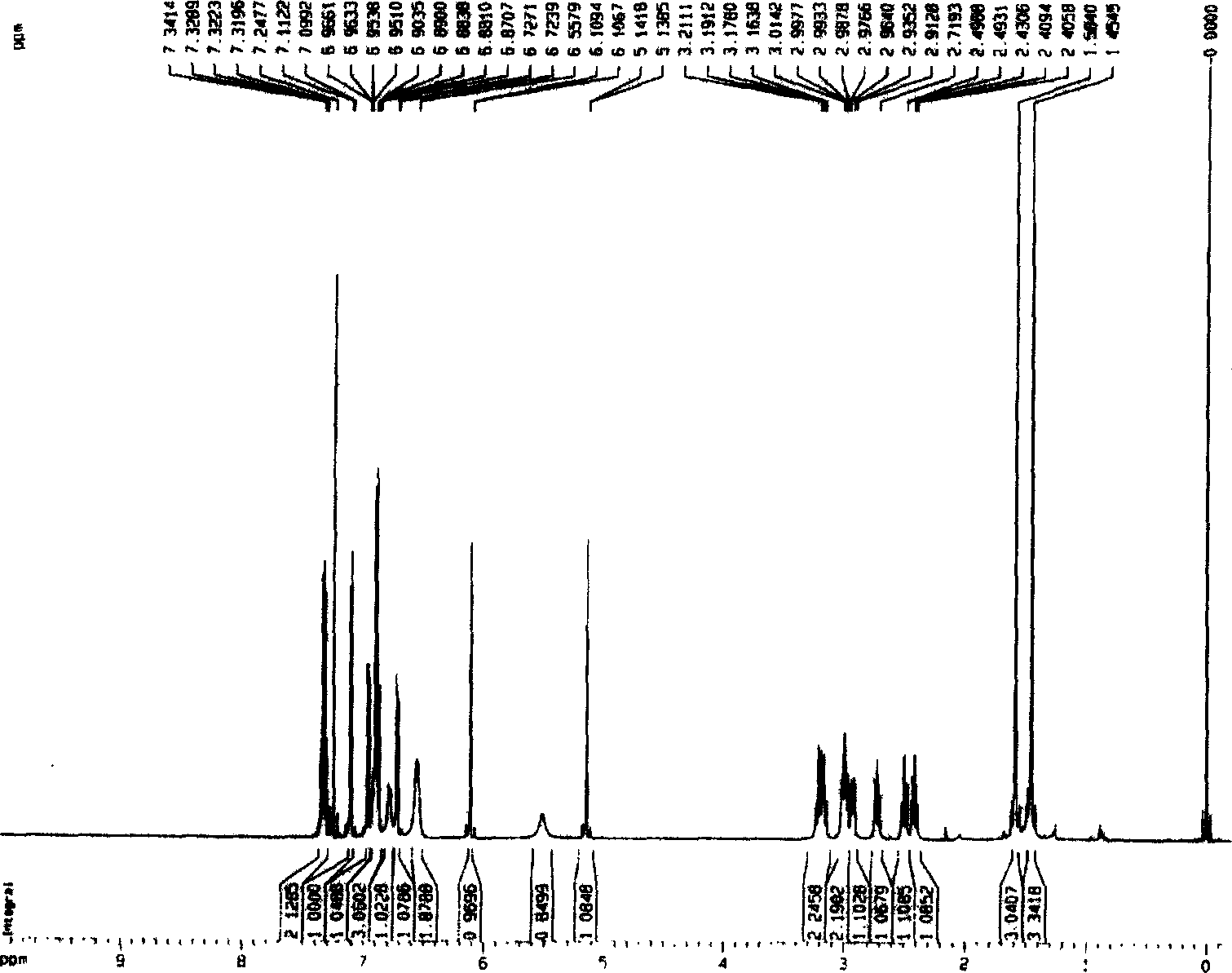
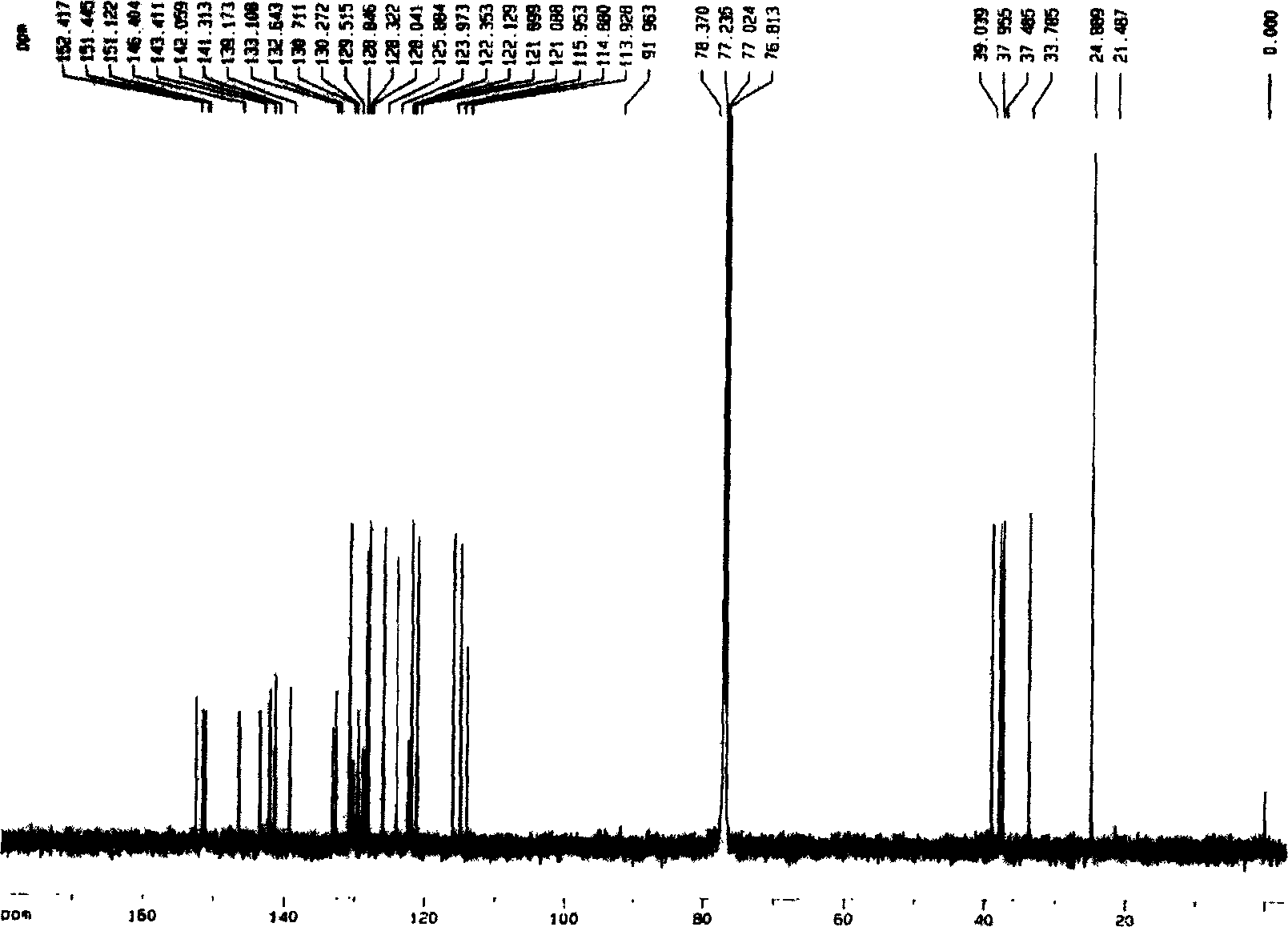
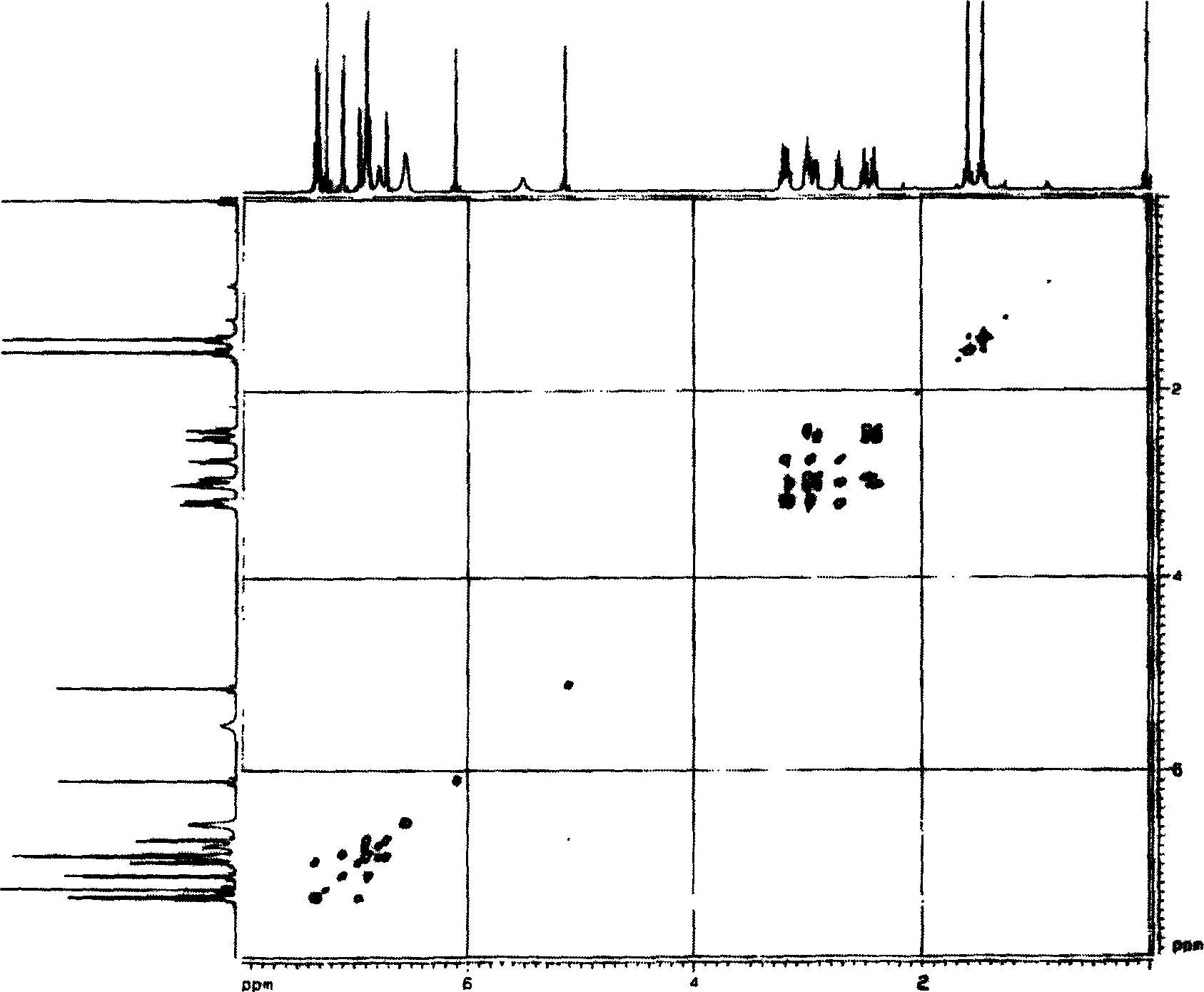
Bibenzil compound 13, 13'-O-iso-propylidene riccardia D and its extraction and separation method and use
A technology of isopropylidene and phylloxane, which is applied in the direction of effective components of heterocyclic compounds, organic chemistry, antifungal agents, etc., can solve the problems of unseen and few antifungal compounds, and achieve strong antifungal effect, novel structure, The effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The ground money (Marchantia polymorpa L.) collected from the area of 1000-1500 meters above sea level in Mount Emei, Sichuan Province was naturally dried and crushed to 8.95Kg, soaked in 8 times the volume of ground money in ether at 28°C for 5 days, filtered under normal pressure, collected ether and extracted Liquid I, repeated 4 times, combined extract I. Soak the medicinal dregs after the last extraction with 3 times the amount of methanol at 28°C for 4 times, each time for 3 days, filter under normal pressure, collect the methanol extracts, combine them, and concentrate under reduced pressure (vacuum degree 0.09Mpa) in a rotary evaporator at 60°C To obtain the methanol extract, extract the extract 3 times with ether equivalent to 3 times the amount of the extract, collect and combine the ether extract II, combine the two parts of the ether solution I and II, and then distill at 60°C to a fluid extract. Evaporate to dryness to obtain the total ether extract. Mix...
Embodiment 2
[0077]Naturally dry and crush Marchantia polymorpa L. collected from Emei Mountain, Sichuan Province, at an altitude of 1000-1500 meters to obtain 9.50Kg, soak in ether at 23°C for 4 days at 5 times the volume of Marchantia, filter under normal pressure, and collect ether for extraction. Solution I, repeated 6 times, combined extract I; soaked the medicinal dregs after the last extraction with 2 times the amount of methanol at 35°C for 5 times, each time for 2 days, filtered under normal pressure, collected methanol extracts, combined, and used a rotary evaporator Concentrate at 70°C under reduced pressure (vacuum degree 0.09Mpa) to obtain the methanol extract, extract the extract 4 times with ether equivalent to 2 times the amount of the extract, collect and combine the ether extract II, and combine the two parts of the ether solution I and II Afterwards, it was distilled at 70°C to a liquid extract, and evaporated to dryness naturally to obtain the total ether extract. Mix t...
Embodiment 3
[0079] Naturally dry and crush the picked Marchantia polymorpa L. to obtain 8.25Kg, soak 10 times the volume of Marchantia polymorpa in ether at 35°C for 7 days, filter under normal pressure, collect the ether extract I, repeat 3 times, and combine the extract I . Soak the medicinal dregs after the last extraction with 4 times the amount of methanol at room temperature for 3 times, each time for 2 days, filter under normal pressure, collect the methanol extracts, combine them, and concentrate on a rotary evaporator at 50°C under reduced pressure (vacuum degree 0.09Mpa) to obtain For the methanol extract, extract the extract twice with ether equivalent to 5 times the amount of the extract, collect and combine the ether extract II, combine the two parts of the ether solution I and II, and distill at 50°C to a liquid extract, and let it evaporate naturally. Dry to obtain the total ether extract. Mix the above-mentioned ether total extract with 3 times the amount of 300 mesh sili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com