Method for preparing cobalt ferrite by coprecipitation
A technology of cobalt ferrite and co-precipitation method, which is applied in the field of preparing nano-cobalt ferrite, can solve problems affecting the application of magnetic materials, and achieve the effects of easy operation and control, good magnetic properties, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
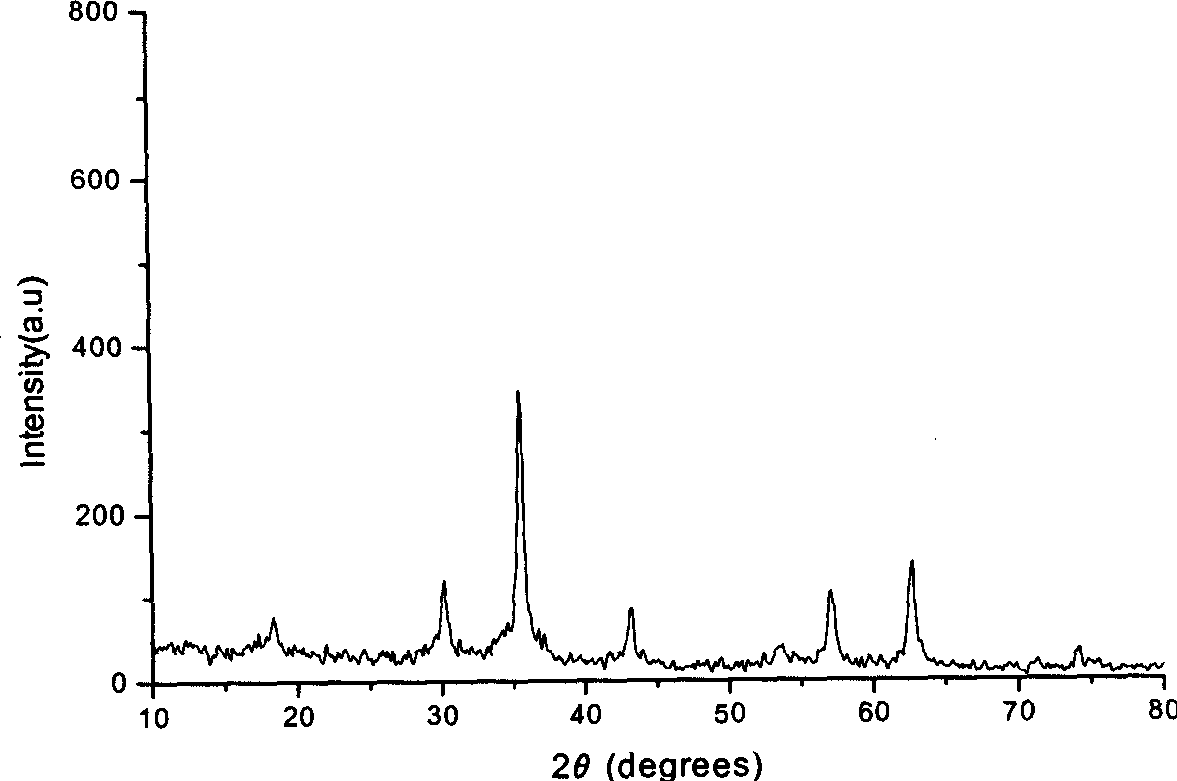
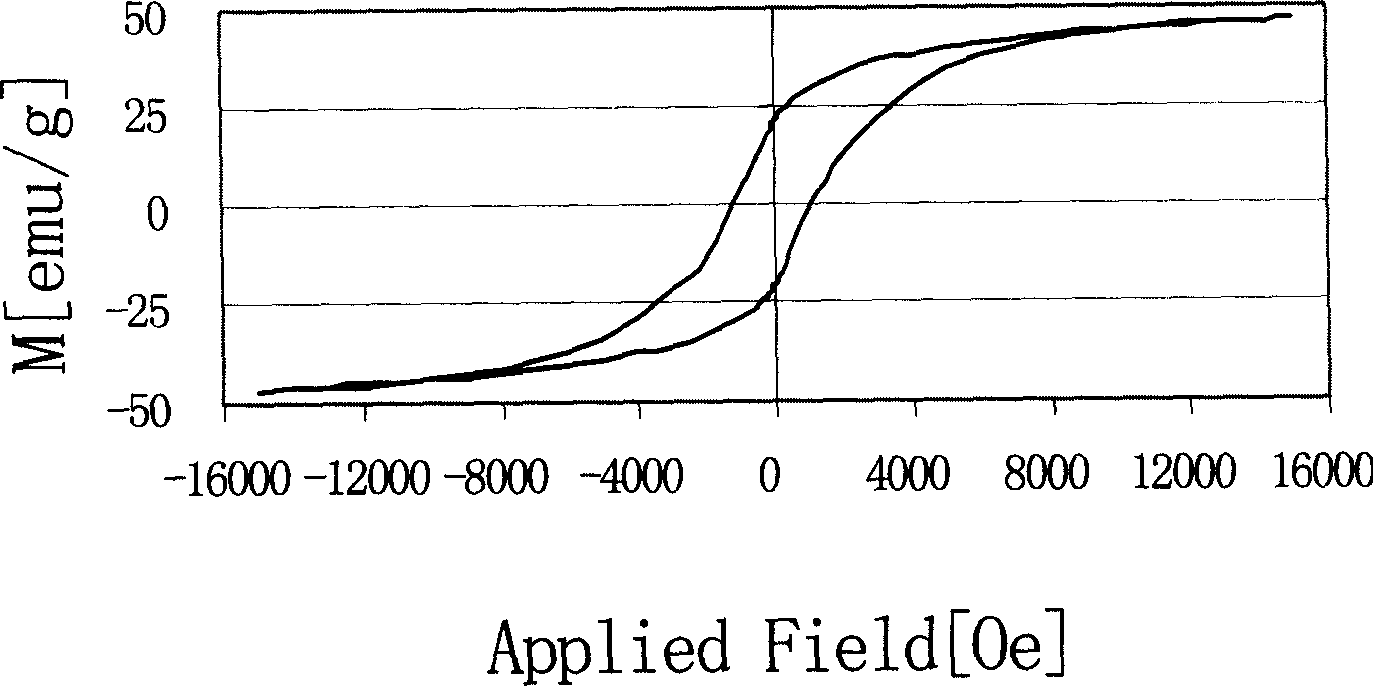
Image
Examples
Embodiment 1
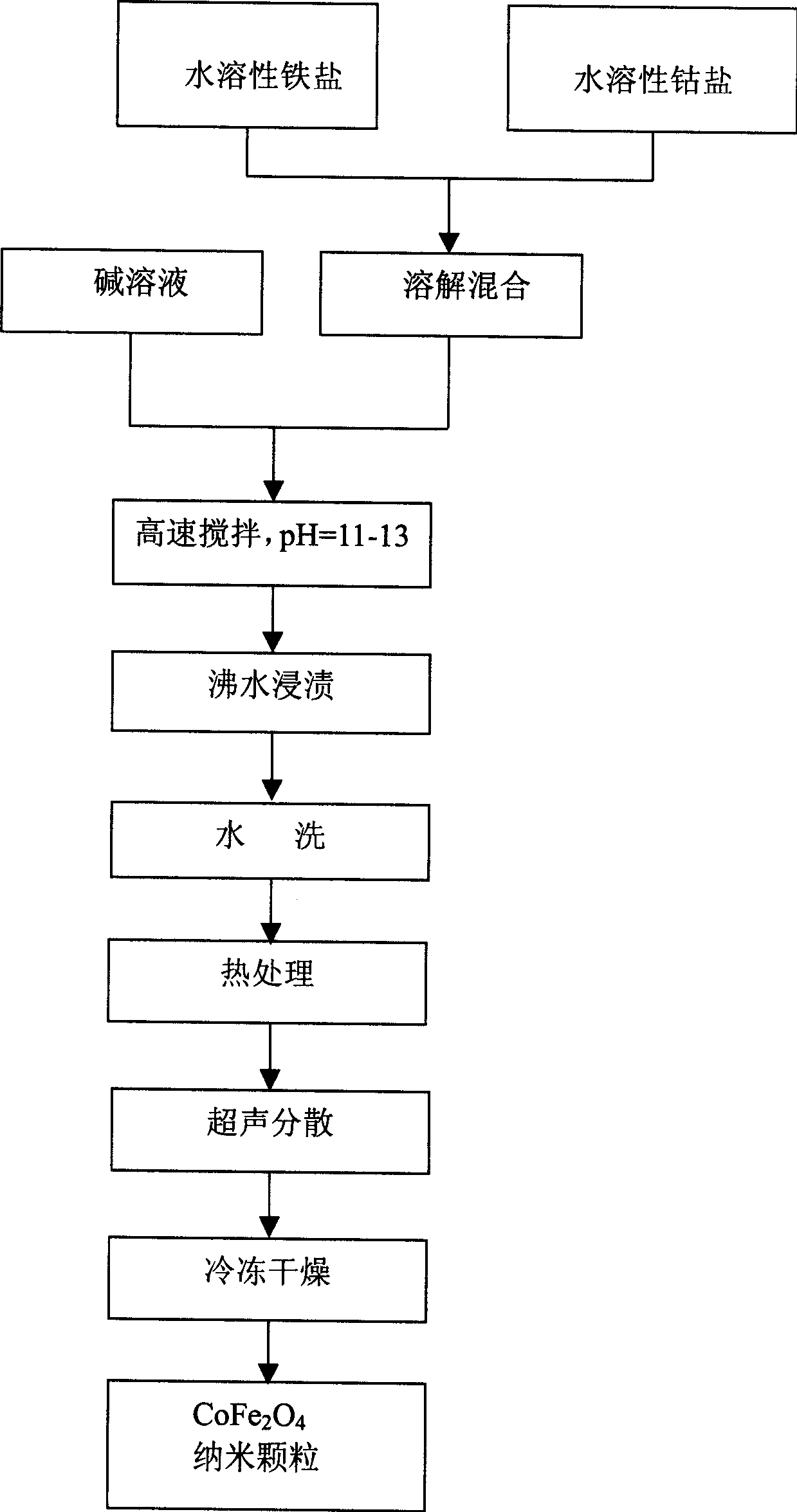
[0026] Co-precipitation method to prepare cobalt ferrite, such as figure 1 As shown, the steps are as follows:
[0027] 1). Weigh 21.6g FeCl 3 ·6H 2 O (0.08mol) and 9.5g CoCl 2 ·6H 2 O (0.04mol) was dissolved in 120ml of distilled water, the Fe / Co molar ratio was 2.0, stirred to obtain a mixed solution, and then the NaOH solution with a concentration of 6.0M was slowly poured under high-speed (3500r / min) stirring to adjust the pH of the solution value to 12.0, after stirring for 30min, a suspension A was formed;
[0028] 2). Soak the suspension A obtained in step 1) in a boiling water bath for 2 hours to obtain a precipitate;
[0029] 3). Wash the precipitate obtained in step 2) with deionized water for 15 times until the pH is 7.0, and filter to obtain a brown precipitate;
[0030] 4). Heat-treat the brown precipitate obtained in step 3) at 100°C for 2 hours, add distilled water after cooling and stir to form a suspension B;
[0031] 5). After dispersing the suspension...
Embodiment 2
[0033] Co-precipitation method to prepare cobalt ferrite, such as figure 1 As shown, the steps are as follows:
[0034] 1).Weigh 19.44gFeCl 3 ·6H 2 O (0.072mol) and 9.5g CoCl 2 ·6H 2 O (0.04mol) was dissolved in 100ml of distilled water, the Fe / Co molar ratio was 1.8, stirred to obtain a mixed solution, and then the KOH solution with a concentration of 5.0M was slowly poured under high-speed (2500r / min) stirring to adjust the pH of the solution value to 13.0, after stirring for 30min, a suspension A was formed;
[0035] 2). Soak the suspension A obtained in step 1) in a boiling water bath for 3 hours to obtain a precipitate;
[0036] 3). Wash the precipitate obtained in step 2) with deionized water for 10 times until the pH is 8.0, and filter to obtain a brown precipitate;
[0037] 4). Heat-treat the brown precipitate obtained in step 3) at 100°C for 3 hours, add distilled water after cooling and stir to form a suspension B;
[0038] 5). After dispersing the suspension ...
Embodiment 3
[0040] Co-precipitation method to prepare cobalt ferrite, such as figure 1 As shown, the steps are as follows:
[0041] 1).Weigh 23.76gFeCl 3 ·6H 2 O (0.088mol) and 9.5g CoCl 2 ·6H 2 O (0.04mol) was dissolved in 150ml of distilled water, the molar ratio of Fe / Co was 2.2, stirred to obtain a mixed solution, then slowly poured ammonia water under high-speed (3500r / min) stirring, adjusted the pH value of the solution to 11.0, and stirred for 60min After that, a suspension A is formed;
[0042] 2). Soak the suspension A obtained in step 1) in a boiling water bath for 1 hour to obtain a precipitate;
[0043] 3). Wash the precipitate obtained in step 2) with deionized water for 20 times until the pH is 6.0, and filter to obtain a brown precipitate;
[0044]4). Heat-treat the brown precipitate obtained in step 3) at 100°C for 1 hour, add distilled water after cooling and stir to form a suspension B;
[0045] 5). After dispersing the suspension B obtained in step 4) in a powerf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
| Residual magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com