Control of ES cell self renewal and lineage specification, and medium therefor
A culture medium and cell technology, applied in the direction of embryonic cells, cell culture active agents, culture process, etc., can solve the problems of pollution, blocking the development of ES cells and their progeny, and achieve the effect of reducing differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Example 1 Method of Culturing ES Cells in a Serum-Free and Feeder-Free Defined Medium
[0104] N2B27 (DMEM / F12 medium supplemented with modified N2 (25 μg / ml insulin, 100 μg / ml apotransferrin, 6 ng / ml luteinizing hormone, 16 μg / ml putrescine, 30 nM sodium selenite and 50 μg / ml bovine serum albumin) and a 1:1 mixture of B27-supplemented Neurobasal Medium)
[0105] ES cells were cultured in N2B27 medium supplemented with LIF (100 U / ml) and BMP4 (100 ng / ml) in a culture dish coated with 0.1% gelatin. For passaging, detach cells with standard protein-free cell dissociation buffer.
[0106] Cells were seeded at a density of approximately 1-5 x 10 4 / cm 2 .
[0107] At the beginning of culture, SU5402 (5 μM) was supplemented in the medium to inhibit differentiation. Cells were transferred to SU5402-free medium after 2 passages.
[0108] ES cells were maintained under these serum-free conditions for 20 passages over 3 months. Cells are typically passaged every 2-4 days,...
Embodiment 2
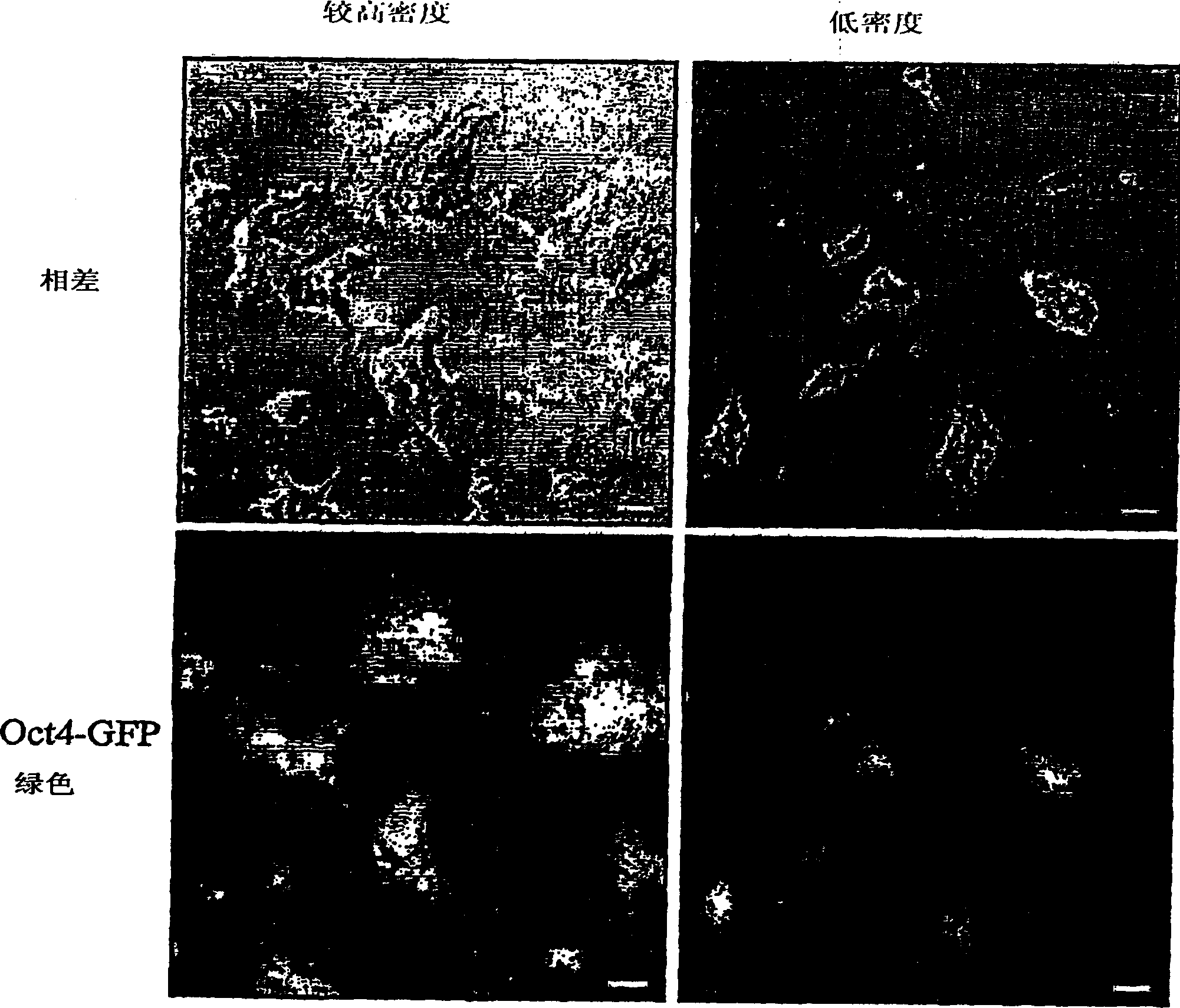
[0110] Example 2 maintains Oct4-GFP expression in ES cells cultured under serum-free conditions
[0111] Oct4GFP (clone C1 ) ES cells were cultured in N2B27 (see Example 1) supplemented with LIF and SU5402 in 0.1% gelatin coated plates. After 2 passages, cells were cultured in N2B27 medium supplemented with LIF and BMP4. After another 2 passages, light microscope images of the cells were taken under phase contrast conditions showing morphology and UV fluorescence to reveal GFP expression. Figure 1 shows the microscope images.
[0112] What is clear is the morphology and expression of GFP, indicating that ES cells maintain their pluripotent phenotype after 4 passages in serum-free medium.
Embodiment 3
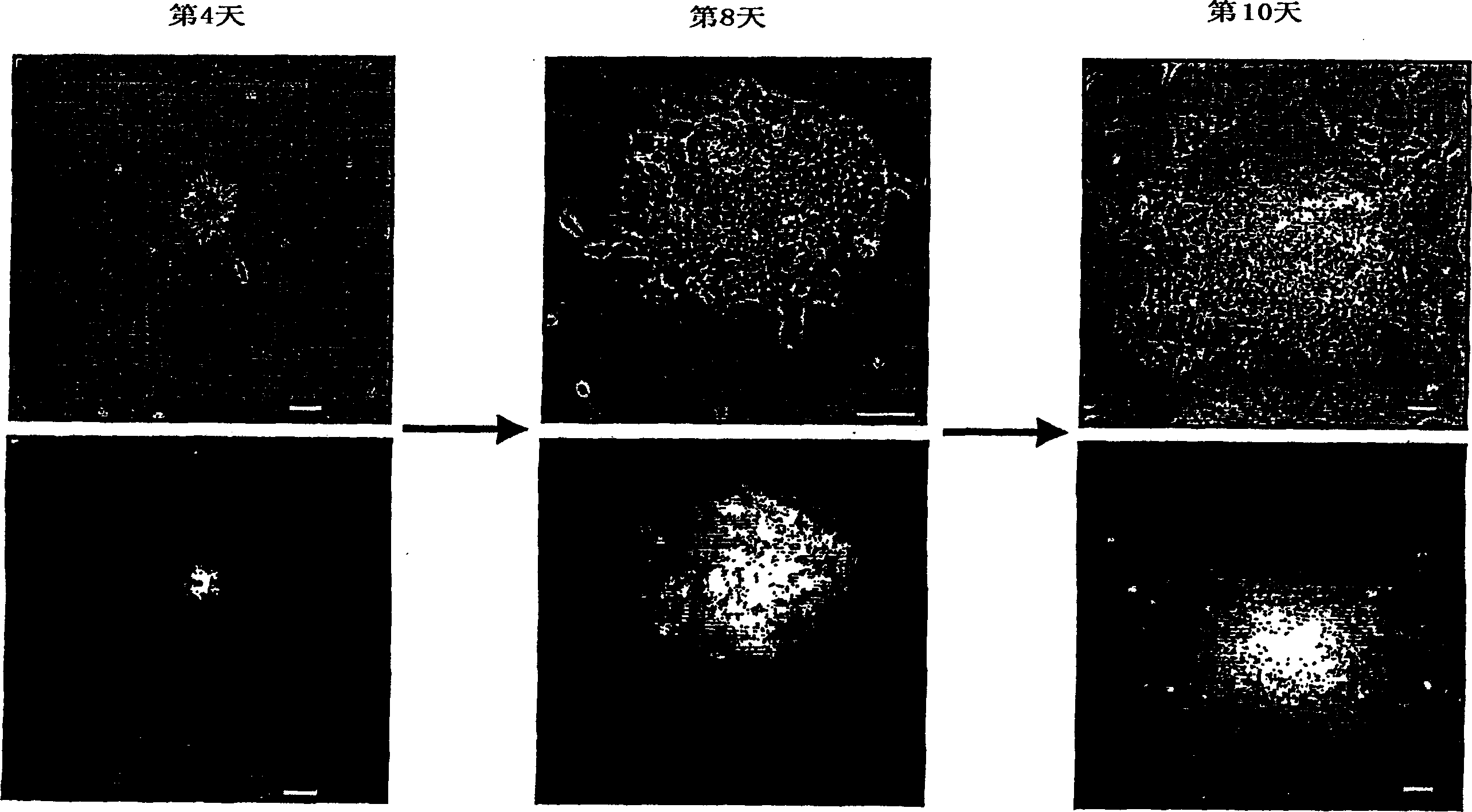
[0113] Example 3 Stable transfection of ES cells
[0114] E14TG2A ES cells were cultured in DMEM F12+ neural basal medium supplemented with N2-B27 supplement and LIF, BMP4. Cells were propagated in 0.1% gelatin-coated plates, harvested and transfected with pPCAG-tauGFP-IP electroporation. transfected cells at 10 5 -10 6 The density per dish was reseeded on 10 cm diameter Petri dishes. After 24 hours, 0.5 μg / ml puromycin was added to select positive clones.
[0115] Between 8-10 days thereafter, single GFP-positive clones were picked into individual wells of 96-well plates, and cells were cultured in the same medium as described above.
[0116] GPF green fluorescence indicates stable transfection of ES cells, and expansion of morphologically undifferentiated ES cells is observed, as shown in figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inoculation density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com