Angio-immunotherapy
An angiogenic and immunogenic technology, applied in gene therapy, antibody medical components, botanical equipment and methods, etc., can solve the problems of inability to clear micrometastasis lesions and shrink tumor volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1. Mouse and Murine Cell Lines
[0067] mice. 4-6 week old C57BL / 6 mice (H-2b) and C3H / HeN mice (H-2k) were from Jackson Laboratory, Bar Harbor, ME. In conducting the research in this article, the researchers complied with the "Guidelines for the Care and Use of Laboratory Animals" proposed by the Laboratory Animal Resources Commission on Life Sciences, National Research Council. for the Care and Use of Laboratory Animals). The Duke University Animal Farm is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
[0068] cell line. The C57BL / 6-derived B16 melanoma F10.9 clone is a highly metastatic, low immunogenic and low class I expression cell line 16 . EL4 is a thymoma cell line (C57BL / 6, H-2b). The murine MBT-2 cell line is derived from a carcinogen-induced bladder tumor in C3H mice 17 , provided by Dr. T. Ratliff (Washington University, St. Louis, MO). The SV40 transformed B6 fibroblast cell line, BLK.SV(TIB-88), ...
Embodiment 2
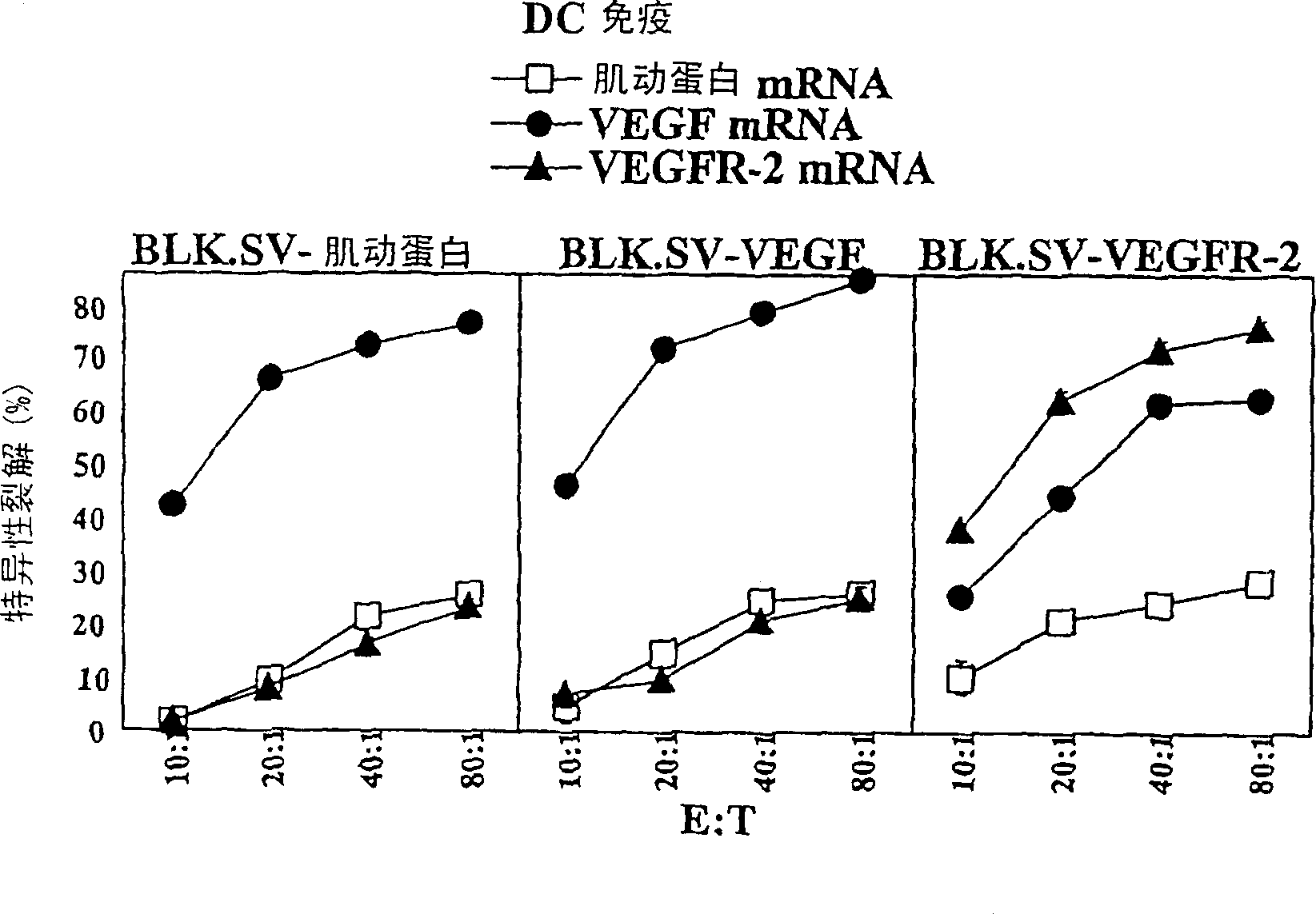
[0069] Example 2. Preparation of dendritic cells transfected with RNA
[0070] BMDCs (Bone Marrow Progenitor-Derived Dendritic Cells) were generated from bone marrow precursor cells as previously described18. Briefly, bone marrow from tibias and femurs of C57BL / 6 mice was collected, and precursor cells were treated with ammonium chloride Tris buffer at 37°C for 3 minutes to deplete red blood cells. Precursor cells were seeded in RPMI-5% FCS supplemented with GM-CSF (15 ng / ml) and IL-4 (10 ng / ml, Peprotech (Rocky Hill, NJ)). cells with 10 6 / ml inoculated and cultured at 37°C and 5% CO 2 condition. After 3 days, floating cells (mainly granulocytes) were removed and adherent cells were supplemented with fresh medium containing GM-CSF and IL-4. After 4 days, non-adherent cells (immature DCs on day 7) were harvested, washed and incubated with 10 6 / ml was inoculated in the medium containing GM-CSF and IL-4. After 1 day, non-adherent cells were harvested, washed, and electrop...
Embodiment 3
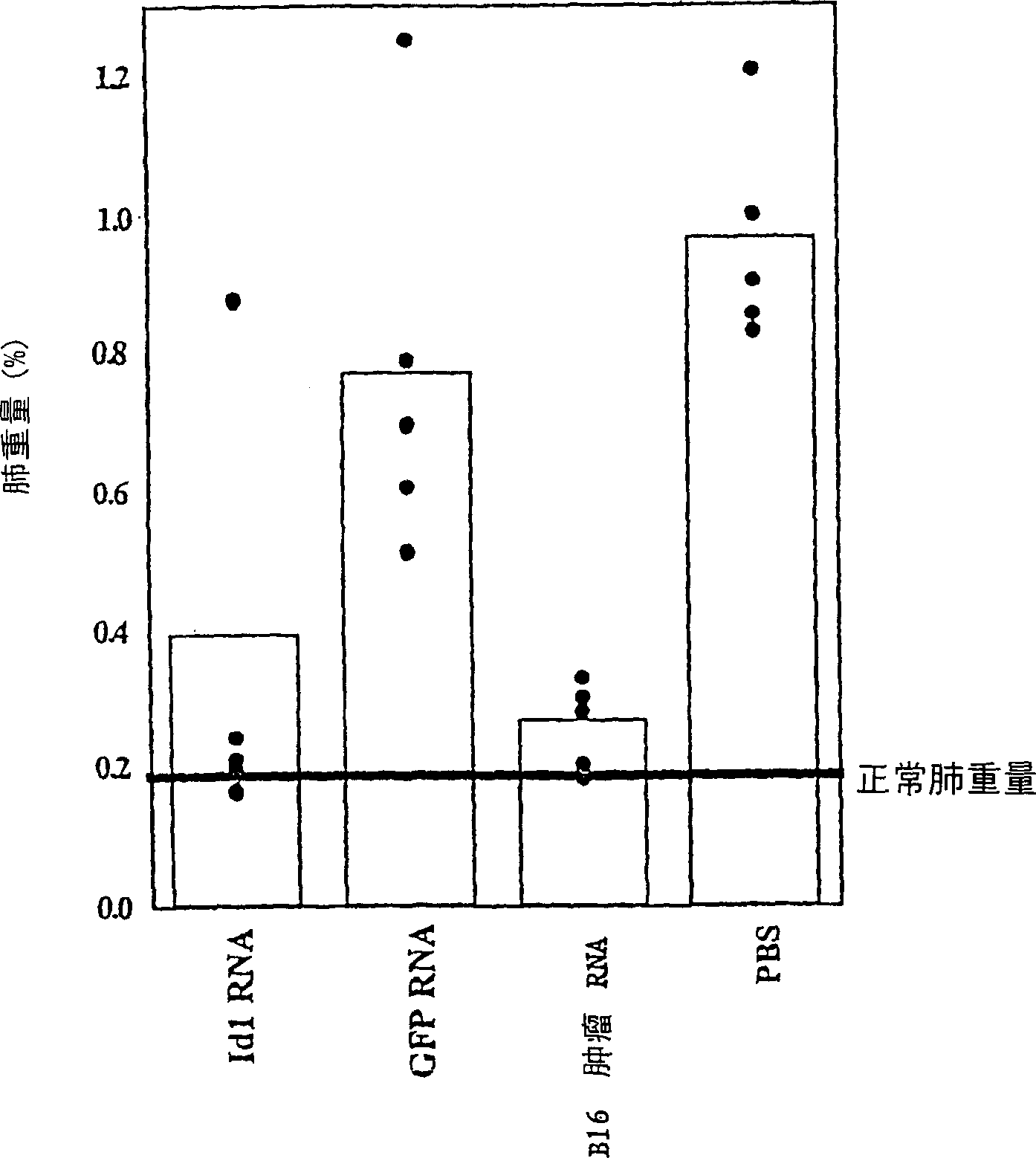
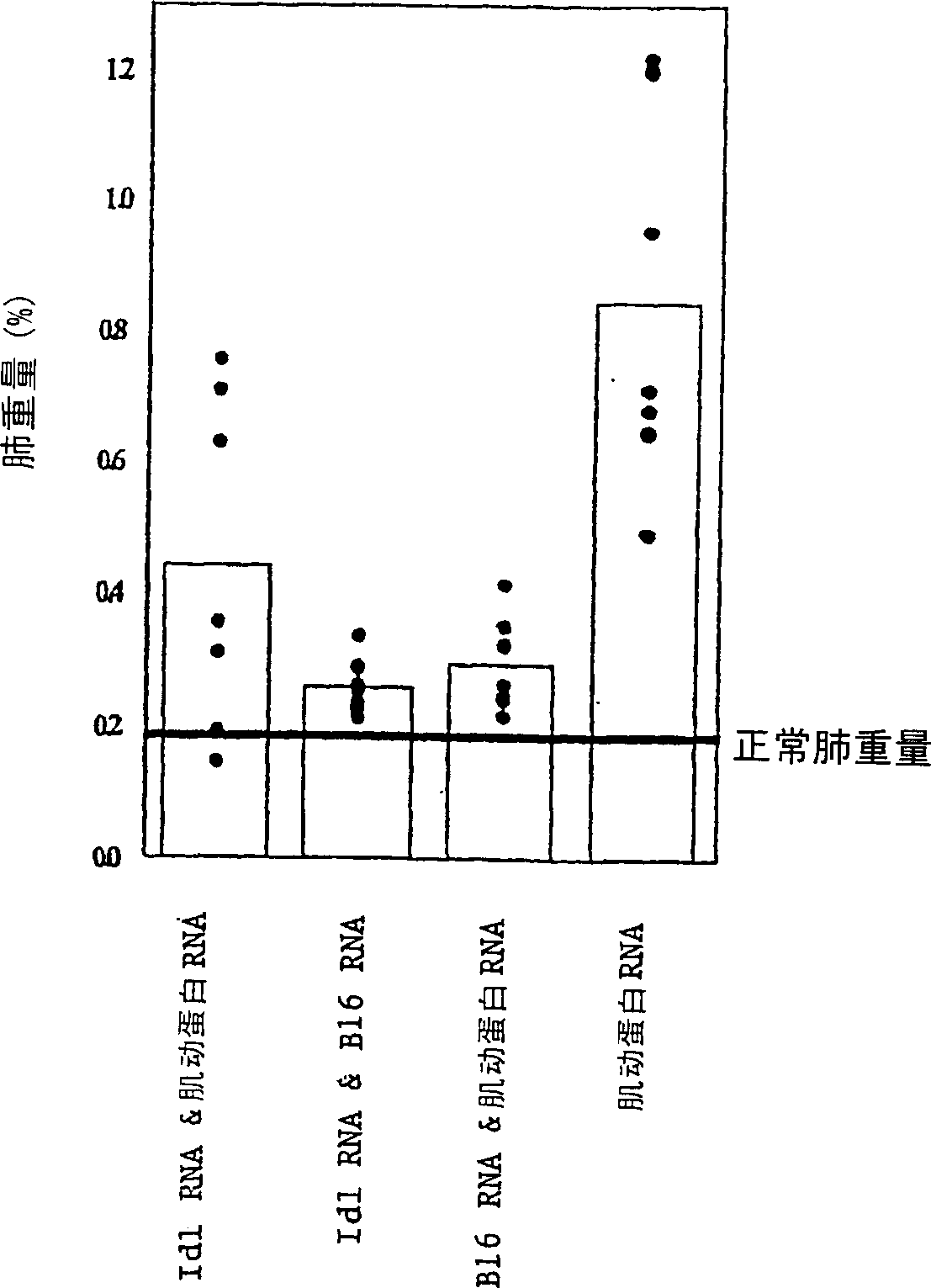
[0073] Example 3. Tumor attack model
[0074] B16 / F10.9 melanoma model: DCs were transfected with various RNA preparations, and the naive syngeneic mice were given intravenous immunization, 5×10 mice per mouse 5 Precursor cell-derived DC (in 200 μl PBS), a total of 3 times, 7 days apart each time. 8-10 days after the last immunization, inject 5×10 intravenously 4 Mice were challenged with F10.9 cells. Mice were sacrificed when the control group died from metastatic cancer. The burden of metastatic cancer was determined by weighing the lungs.
[0075] MBT-2 mouse bladder tumor model: DCs were transfected with various RNA preparations, and the original syngeneic mice were given intravenous immunization, each mouse 5×10 5 Precursor cell-derived DC (in 200 μl PBS), a total of 3 times, 7 days apart each time. 8-10 days after the last immunization, inject 2-5×10 subcutaneously (side abdomen) 5 MBT-2 cells challenged mice. Beginning on day 6, tumor growth was measured every o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com