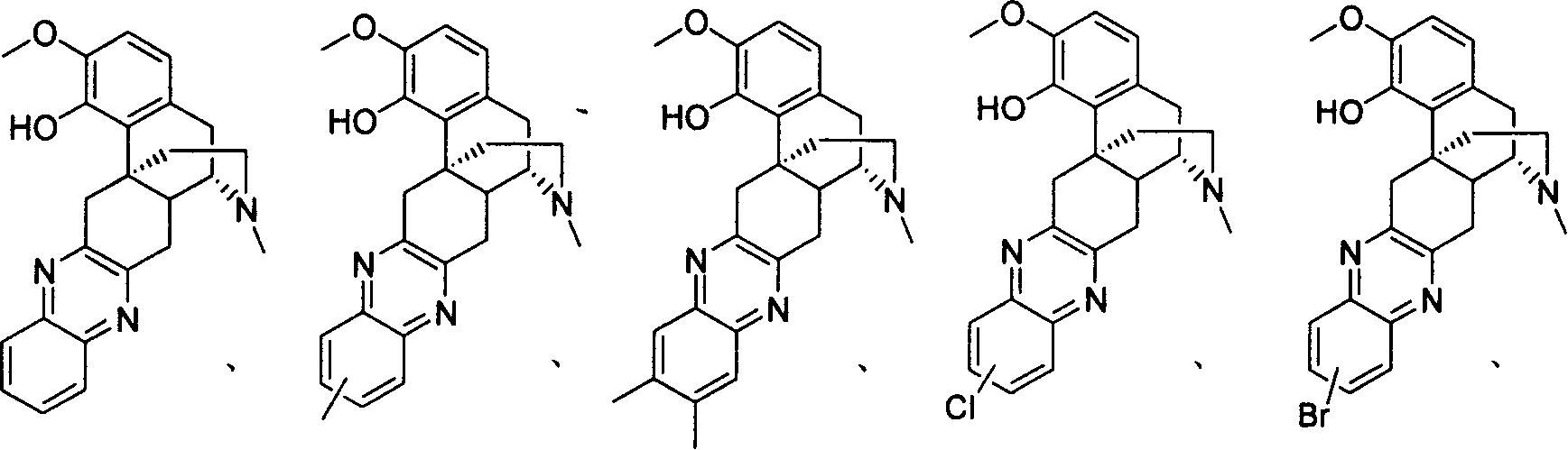
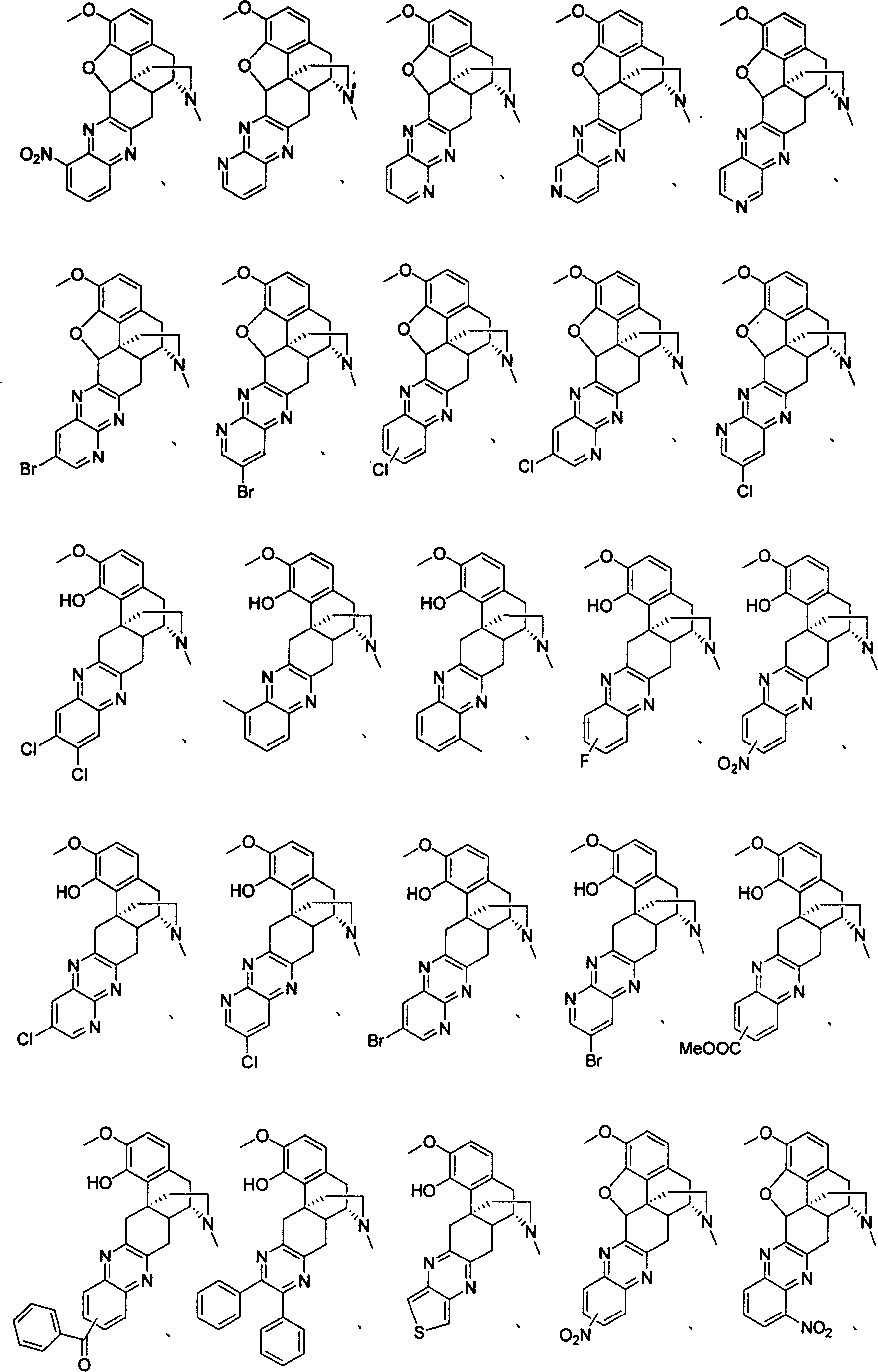
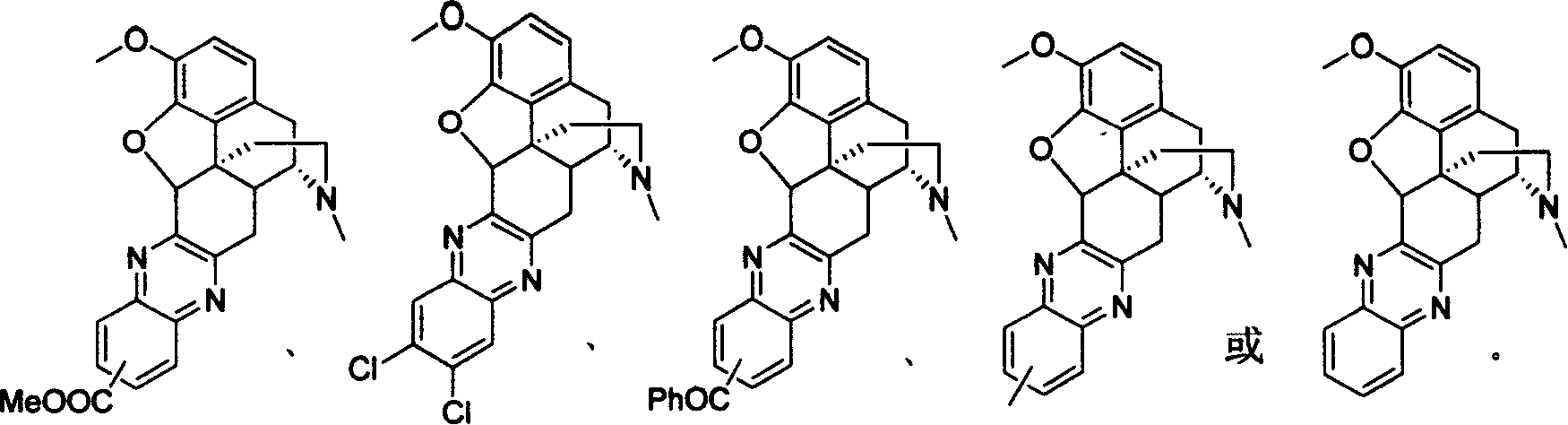
Derivative of sinomenine with pyrazinc cyclc being connected to C cycle, synthetic method and application
A synthesis method and technology of sinomenine are applied in the fields of sinomenine derivatives with pyrazine ring connected to C ring, synthesis and use thereof, and can solve the problems of large dosage and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] The chloroform solution of equivalent o-phenylenediamine was added dropwise in the chloroform solution of 0.73g compound 1 (2.3mmol), stirred at room temperature for 5 hours, the reaction solution was concentrated to dryness, and chloroform-petroleum ether recrystallized to obtain 0.65g white solid ( Compound 2), yield 78%.
[0052] 1 H NMR (CDCl 3 , 300MHz): 8.00(1H, m), 7.87(1H, m), 7.61(2H, m), 6.71, 6.61(2H 2d,, J=4.8), 6.07(1H, Brs.,), 5.02(1H, d, J=17.1), 3.71(3H, s), 2.87~3.27(6H, m), 2.58(2H, m), 2.48(3H, s), 2.21(1H, dt, J=2.7, 12.0), 2.10 (1H, J=12.9) 1.92 (1H; dt, J=4.2, 12.6) ppm.
[0053] 13 C NMR (CDCl 3 : CD 3 OD=10∶1,75MHz):153.588,153.264,144.728,144.317,140.826,140.539,130.219,128.910,127.572,127.472,122.279,118.322,109.079,56.270,55.580,46.563,43.712,42.320,41.961,41.914,37.338 , 35.794, 32.903, 22.957ppm.
[0054] IR (KBr): 2932, 1602, 1483, 1400, 1356, 1276, 1228, 1066, 1053, 836, 761 cm-1 MS (EI, m / z): 387 (M + ).
[0055] HRM...
Embodiment 2
[0057]
[0058] An equivalent amount of 3,4-diaminotoluene in chloroform was added dropwise to 1.0 g of compound 1 (3.17 mmol) in chloroform, and stirred at room temperature for 8 hours. The reaction solution was concentrated and separated by column chromatography to obtain 0.9 g of a white solid with one point in TLC, and the yield was 70%. However, the mixture of compounds 3 and 4 was identified by proton nuclear magnetic resonance, and the ratio was compound 3:compound 4 (or compound 4:compound 3)=6:1. It cannot be determined whether compound 3 or compound 4 has the larger amount.
[0059] 1 H NMR (CDCl 3 , 300MHz) (whichever is more in the mixture): 1 H NMR (CDCl 3 , 300MHz): 7.71(2H, m), 7.40(1H, d, J=8.4), 6.60, 6.56(2H, 2d,, J=8.1), 6.30(1H, Brs.,), 5.02(1H, d , J=17.5), 3.67(3H, s), 2.87~3.27(6H, m), 2.55-2.69(2H, m), 2.53(3H, s), 2.48(3H, s), 2.21(1H, dt, J = 2.7, 12.6), 2.1 (1H, d, J = 12.9) 1.91 (; 1H, dt, J = 3.9, 12.0).
[0060] MS (EI, m / z): 401 (M + )...
Embodiment 3
[0062]
[0063] 1.15 g of compound 1 (3.67 mmol) and an equivalent of 4,5-dimethyl-o-phenylenediamine were dissolved in dichloromethane, stirred at room temperature for 5 hours, concentrated and separated by column chromatography to obtain 1.21 g of a white solid (compound 5), Yield 79%.
[0064] 1 H NMR (CDCl 3 , 300MHz): 7.55(1H, s), 7.50(1H, s), 6.57(2H 2d, J=8.3), 6.06(1H, Brs.,), 5.02(1H, d, J=17.0), 3.62( 3H, s), 2.92~3.16(6H, m), 2.55(2H, m), 2.46(3H, s), 2.36(3H, s), 2.31(3H, s), 2.22(1H, dt, J=3.0, 12.9 ), 2.1 (1H, d, J=12.9) 1.91 (1H, dt, J=4.8, 13.2)
[0065] 13 C NMR (CDCl 3 ,75.0MHz)152.777,152.411,145.022,144.637,140.396,140.113,138.829,138.768,131.268,127.657,127.234,123.736,118.701,108.954,77.637,77.215,76.795,56.706,55.904,47.037,44.576,43.109,43.009, 38.327, 36.324, 33.550, 23.407, 20.279, 20.245 ppm.
[0066] IR (KBr): 3005, 2916, 2802, 1604 1483, 1439, 1335, 1274, 1228, 1153, 1104, 1066, 1054, 1023, 989, 867, 857, 792cm -1 .
[0067] MS(EI): 415...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com