Treatment and prophylaxis with 4-1BB-binding agents
An agonist and B cell technology, applied in gene therapy, anti-inflammatory agents, drug combinations, etc., can solve the problems of long-term treatment and inability to clear autoreactive lymphocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Materials and methods
[0082] mouse
[0083] B6.MRL-Tnfrsf6 lpr , (B6 / lpr), MRL / MpJ-Tnfrsf6 lpr (MRL / lpr), and MRL.129P2(B6)-Tnfsf6 tmlqsa (Fas - / - ) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). C57BL / 6 wild-type (B6 / wt) mice were purchased from the National Cancer Institute (Frederick, MD).
[0084] In vitro antibody treatment
[0085] An agonistic monoclonal antibody (mAb), 2A, against 4-1BB was generated as previously described (Wilcox et al., supra). Rat IgG was purchased from Sigma Chemical Company (St. Louis, MO) and served as a control antibody. Starting at 2-3 months of age, each mouse was injected weekly intraperitoneally (i.p.) with 200 μg / mouse 2A or rat IgG for three weeks.
[0086] Flow Cytometry Analysis
[0087] The following antibodies were purchased from BD-PharMingen (San Diego, CA): Cy-Chrome TM Labeled CD4 (H129.19), R-phycoerythrin (R-PE)-conjugated anti-mouse CD8a (53-6.7), R-PE-conjugated anti-mouse Th...
Embodiment 2
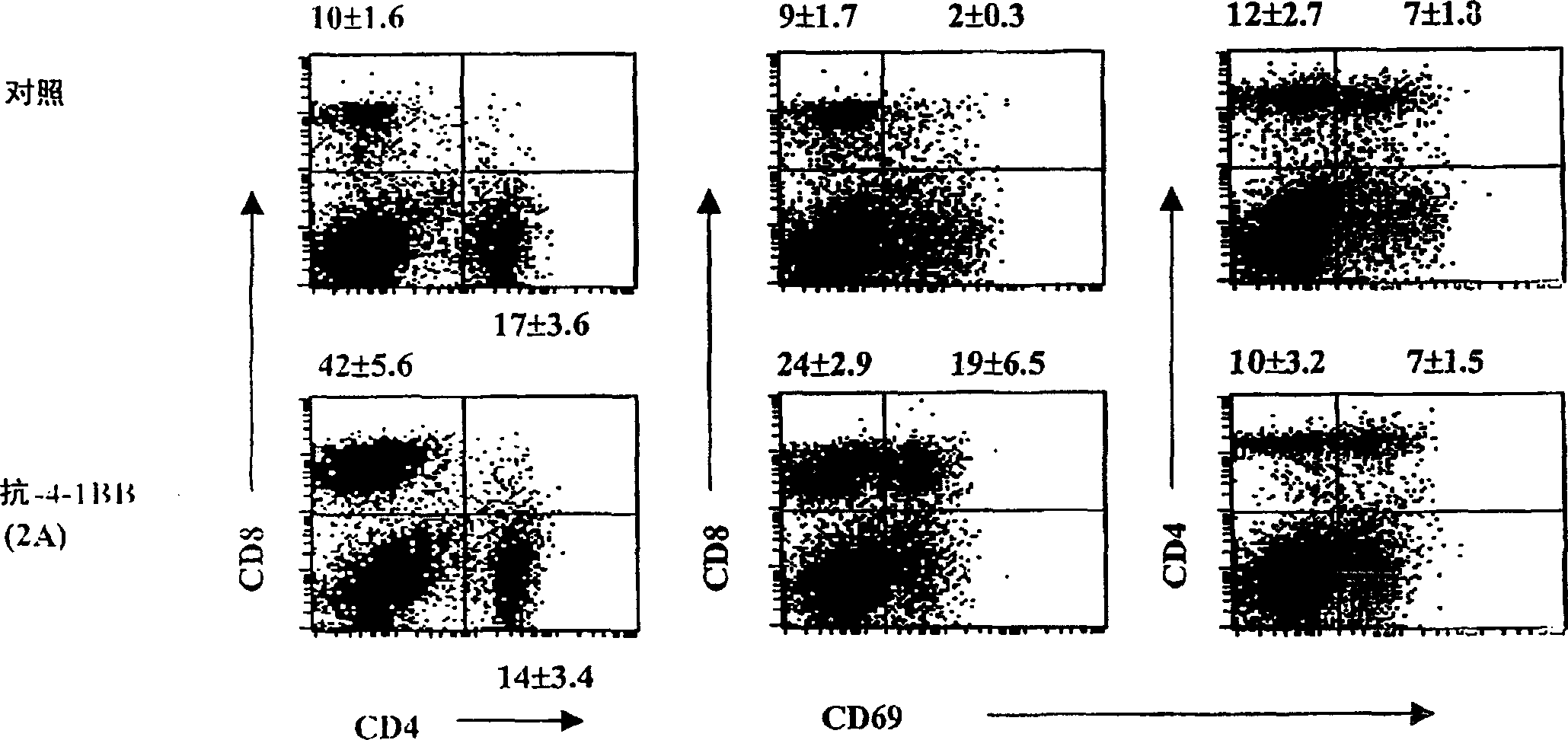
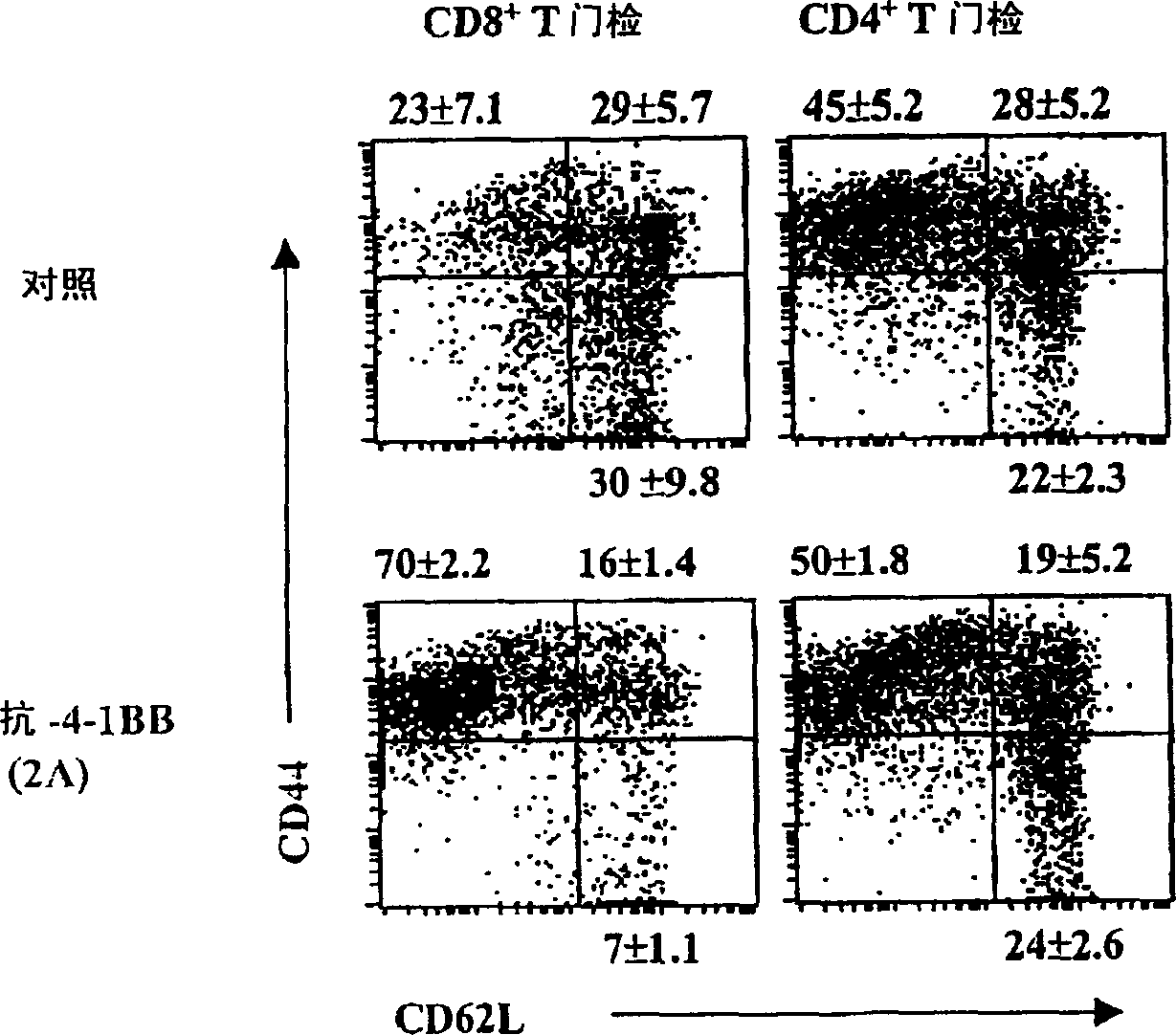
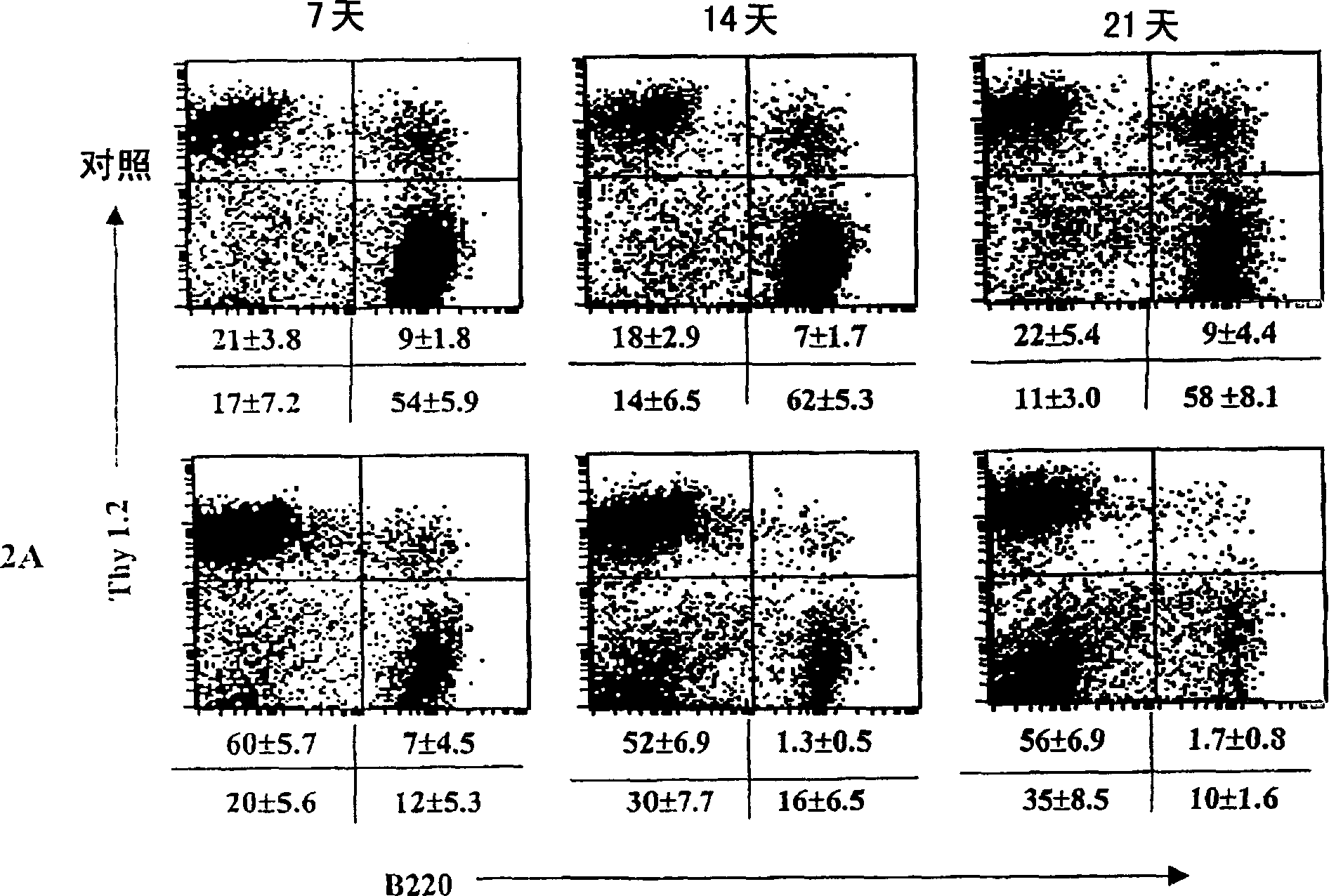
[0105] Example 2: Treatment of B6 / lpr mice with agonistic anti-4-1BB mAb (2A) preferentially activates CD8 + T cells, but reduced DNTC and B cell populations
[0106] B6 / lpr mice are naturally deficient in Fas and develop a lymphoproliferative disorder characterized by accumulation of autoreactive lymphocytes shortly after birth. To investigate the role of 4-1BB signaling in the activation of autoreactive lymphocytes, two- or three-month-old B6 / lpr and B6 / wt mice were treated with agonistic anti-4-1BB mAb (2A) or control rat IgG . Mice were treated at weekly intervals for three weeks, and splenocytes were analyzed by flow cytometry at various time points. Three weeks after the start of treatment, CD8 in spleens of 2A-treated mice compared with control mice + The percentage of T cells increased about 3-4 fold, while CD4 + The percentage of T cells remained unchanged ( Figure 1a on the left). In addition, in 2A-treated mice, CD4 + The number of T cells decreased, while CD...
Embodiment 3
[0108] Example 3: Administration of 2A greatly ameliorated lymphadenopathy in MRL / lpr mice
[0109] Compared to B6 / lpr mice, MRL / lpr mice typically exhibit more severe lymphoproliferative disease at a younger age and truly exhibit lupus-like features. 9-10 week old MRL / lpr mice generally have a significant number of abnormal DNTC and display higher levels of autoantibody IgG anti-DNA in serum. To test whether 2A has a potential therapeutic effect in the treatment of autoimmune diseases, 9-10 week old MRL / lpr mice were treated for three weeks with a weekly dose of 200 μg 2A or control rat IgG. All control mice exhibited progressive severe lymphadenopathy, whereas only two of 10 mice in the 2A-treated group developed 1-2 small palpable lymph nodes (LN) at 5 months of age ( Figure 2a ). In addition, at 4 months of age, 2A-treated mice had considerably smaller spleens and peripheral lymph nodes compared to control mice ( Figure 2b ). In the spleen and peripheral lymph nodes ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com