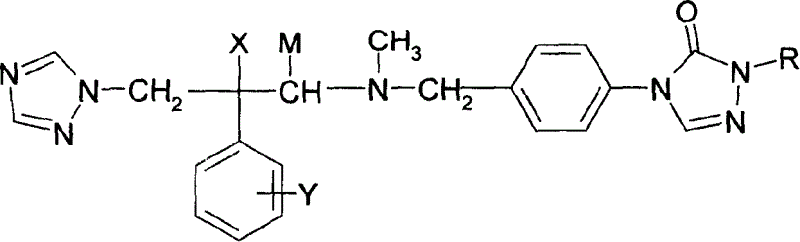
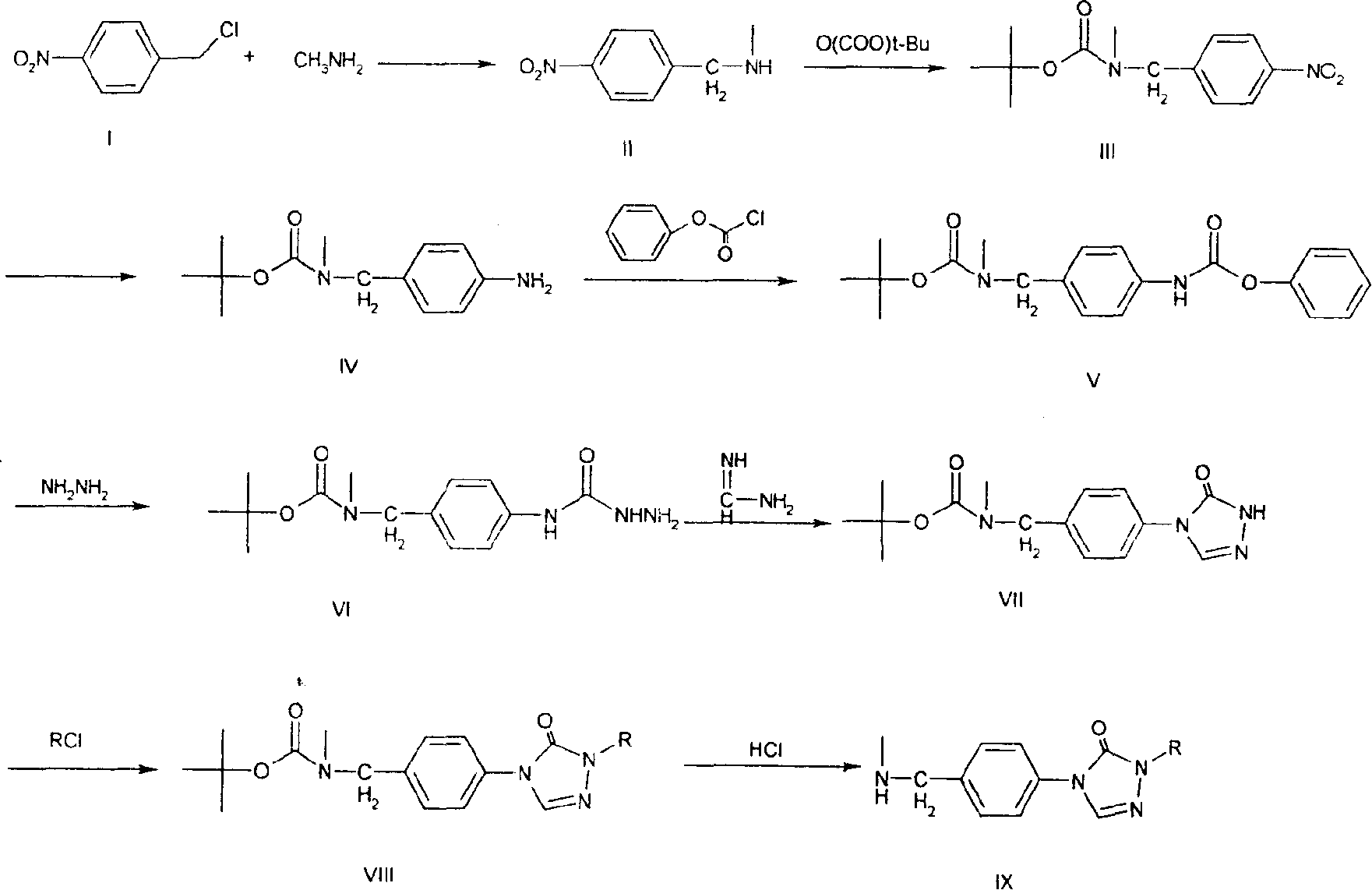
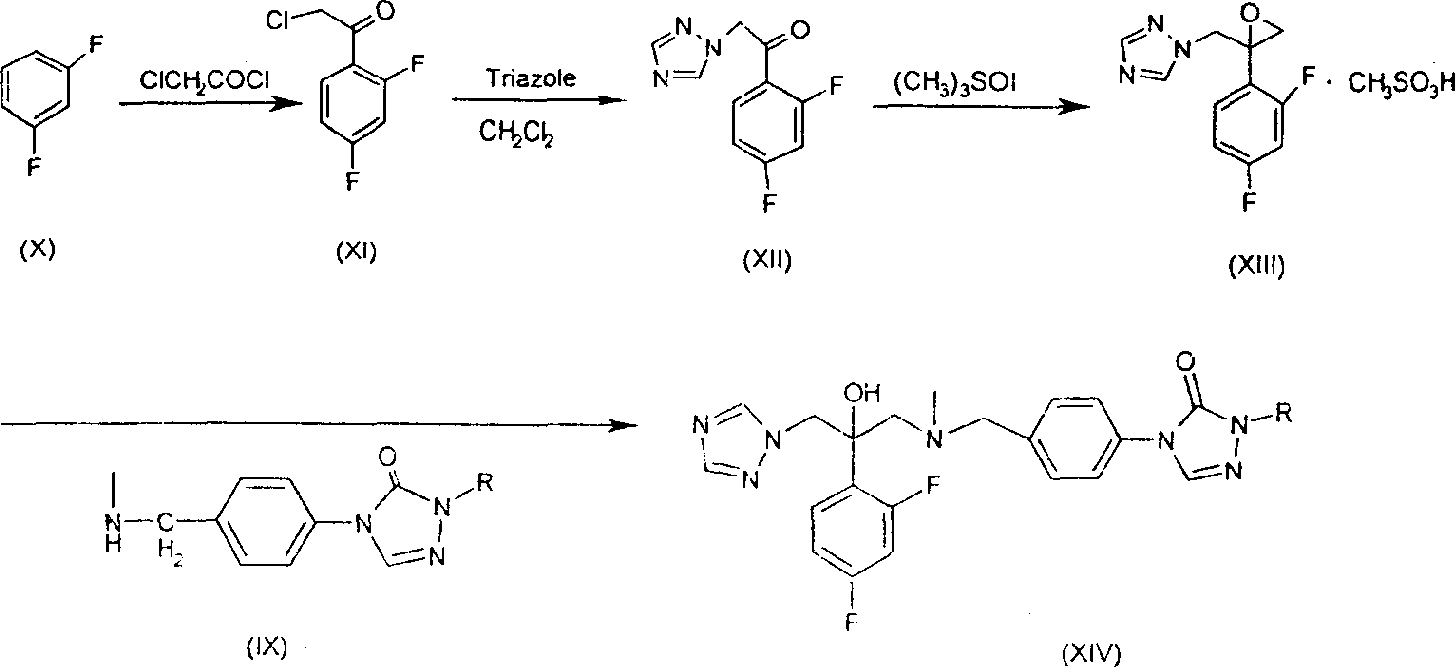
Substituted triazolone benzyl amine triazole antifungal compounds and method for preparing same
A kind of technology of triazolone benzylamine triazole and compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: the preparation of N-methyl p-nitrobenzylamine (II)
[0092] Add 700ml of saturated methylamino alcohol solution and 24g (0.14mol) of p-nitrobenzyl chloride to a 1000ml round-bottomed flask, reflux at 60°C for 5h, the reaction solution turns from green to brownish red, evaporate the solvent to dryness after the reaction, and the residue Disperse in 300ml of dilute hydrochloric acid solution and 100ml of anhydrous ether, adjust the aqueous layer to alkaline with NaOH, extract with anhydrous ether (200ml×3), combine the extracts, anhydrous Na 2 SO 4 After drying and filtering, the filtrate was evaporated to dryness to obtain 21.9 g of solid, with a yield of 92.86%.
Embodiment 2
[0093] Embodiment 2: Preparation of N-methyl-N-tert-butoxycarbonyl-4-nitrobenzylamine (III)
[0094] In a 500ml round bottom flask, add N-methyl-p-nitrobenzylamine (II) 16.7g (0.1mol) and 250mlCH 2 Cl 2 , at 0℃~5℃ (ice bath) slowly drop (BOC) 2 O 22g (0.1mol) dissolved in CH 2 Cl 2 50ml of solution. After the dropwise addition, most of the products were formed. Continue to react at room temperature for 10h, and the reaction is almost complete. Add 300ml of chloroform to dilute, wash with water (200ml×3), wash with saturated brine (200ml×3), anhydrous Na 2 SO 4 Dry, filter, and evaporate the filtrate to obtain 25 g of a brown-red liquid, with a yield of 93.4%.
Embodiment 3
[0095] Embodiment 3: Preparation of N-methyl-N-tert-butoxycarbonyl-4-aminobenzylamine (IV)
[0096] 24g (0.09mol) of compound (III), 96ml of 85% hydrazine hydrate and 300ml of absolute ethanol were placed in a 500ml round bottom flask, and a catalytic amount of active nickel was carefully added. Reflux at 75°C for 10 h. After the reaction, filter (residual nickel was treated with dilute hydrochloric acid), and the filtrate was evaporated to dryness to obtain 21.3 g of a yellow solid with a yield of 100%. Melting point: 105~107℃. It can be used in the next reaction without purification.
[0097] 1HNMR: 6.04~7.03 (4H, m, Ar-H), 4.29 (2H, s, -CH2-Ph), 3.63 (2H, br, -NH 2 ), 2.78 (3H, s, N-CH 3 ), 1.48(9H, s, -C(CH 3 ) 3 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com