Prepn of nickel-base catalyst for decomposing ammonia
A nickel-based catalyst and ammonia decomposition technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of low activity, easy sintering and growth of nickel grains, and difficulty in achieving high conversion of ammonia High activity, cheap raw materials, and easy control of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
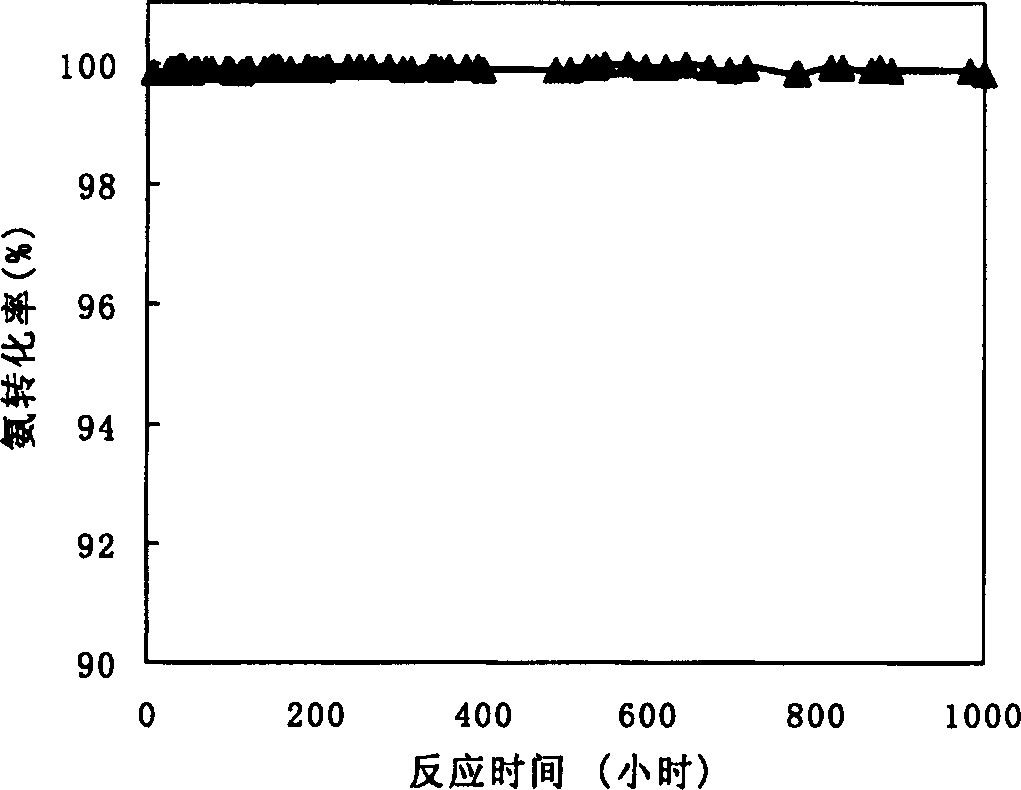
Image
Examples
Embodiment 1
[0017] Dissolve 155g of nickel nitrate and 74g of aluminum nitrate in 500ml of deionized water to prepare a mixed solution for later use. Get stoichiometric ammonium carbonate and dissolve it in 500ml deionized water, and use it as a precipitating agent for subsequent use. The above two solutions were added dropwise into the same vessel under stirring, and the pH value of the solution was controlled to be 8. After the feed was completed, it was stirred and aged for 2 hours, centrifuged and washed with deionized water to obtain a gel. After drying at 120°C, the temperature was programmed to rise to 600°C and baked in air for 8 hours. Prepare Ni / Al with nickel oxide content of 80% 2 o 3 catalyst.
[0018] According to the above method, the feed intake is changed to 117g nickel nitrate and 147g aluminum nitrate to obtain Ni / Al with a nickel oxide content of 63%. 2 o 3 catalyst.
[0019] According to the above-mentioned method, change feeding intake into 7.8g nickel nitrate...
Embodiment 2
[0024] Dissolve 46g of nickel nitrate, 59g of aluminum nitrate, and 9g of lanthanum nitrate in 200ml of deionized water to prepare a mixed solution for later use. Get stoichiometric ammonium carbonate and dissolve it in 200ml deionized water, and use it as a precipitating agent for subsequent use. The above two solutions were added dropwise into the same vessel under stirring, and the pH value of the solution was controlled to be 8. After the feed was completed, it was stirred and aged for 6 hours, centrifuged and washed with deionized water to obtain a gel. After drying at 120°C, the temperature was programmed to rise to 600°C and baked in air for 4 hours. Prepare Ni / La-Al with nickel-aluminum atomic ratio of 1.2 and lanthanum-nickel atomic ratio of 0.22 2 o 3 catalyst.
[0025] According to the above method, the feed intake was changed to 46g of nickel nitrate, 59g of zirconium nitrate, and 11g of cerium nitrate to obtain Ni / Ce-ZrO with a nickel-aluminum atomic ratio of ...
Embodiment 3
[0031] According to the preparation method described in Example 2, ammonium carbonate was changed to ammonium bicarbonate to obtain Ni / La-Al with a nickel-aluminum atomic ratio of 1.2 and a lanthanum-nickel atomic ratio of 0.21 2 o 3 catalyst.
[0032] Take 1.0 g of the catalyst, and perform activity evaluation according to the conditions described in Example 2. Experimental results: when the ammonia gas flow rate is 333ml / min at 550°C, the ammonia gas conversion rate is 99.7%.
[0033] With Ni / La-Al gained in embodiment 2 2 o 3 Compared with the catalyst, the activity of the catalyst prepared by using ammonium bicarbonate or ammonium carbonate as the precipitating agent is equivalent. This shows that both ammonium carbonate and ammonium bicarbonate are better precipitants for the preparation of highly active nickel-based ammonia decomposition catalysts by co-precipitation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com