Phospholipid derivatives of valproic acid and mixtures thereof
A derivative, the technology of valproic acid, is applied in the field of bipolar cell disease and pain, migraine, and epilepsy, and can solve the unmet long-term needs of antiepileptic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0054] Example 1: Synthesis of DP-VPA
[0055] The synthesis of the DP-VPA is a two-stage process. The first stage is aimed at the preparation of valproic anhydride, ie by heating valproic acid in a solution of acetic anhydride in the presence of the catalyst pyridine. In the second stage, DP-VPA is prepared by means of the reaction between valproic anhydride and lysolecithin. The reaction is carried out in valproic anhydride solution at 90-100°C under the catalysis of 4-dimethylaminopyridine.
[0056] The extraction and purification of the product obtained is carried out in four steps. The first stage of purification involves extraction of unreacted valproic anhydride, valproic acid and catalyst (4-aminopyridine) with acetone. The crude product obtained is precipitated from solution and isolated in a second stage. The solid product obtained is washed to remove residual compounds in the third stage. Finally, the product was recrystallized several times from acetone / ethano...
example 2
[0063] Example 2: C16 / C 18 -Synthesis of DP-VPA
[0064] C 16 / C 18 - A mixture of DP-VPA was prepared following the same procedure as described in Example 1 for the preparation of DP-VPA. The difference is that, in the case of mixture compositions, in the interaction of valproic anhydride with lyso-lecithin obtained from soybeans and saturated by hydrogenation (S VPC-3 from the company Lipoid, Ludwigshafen ,Germany).
example 3
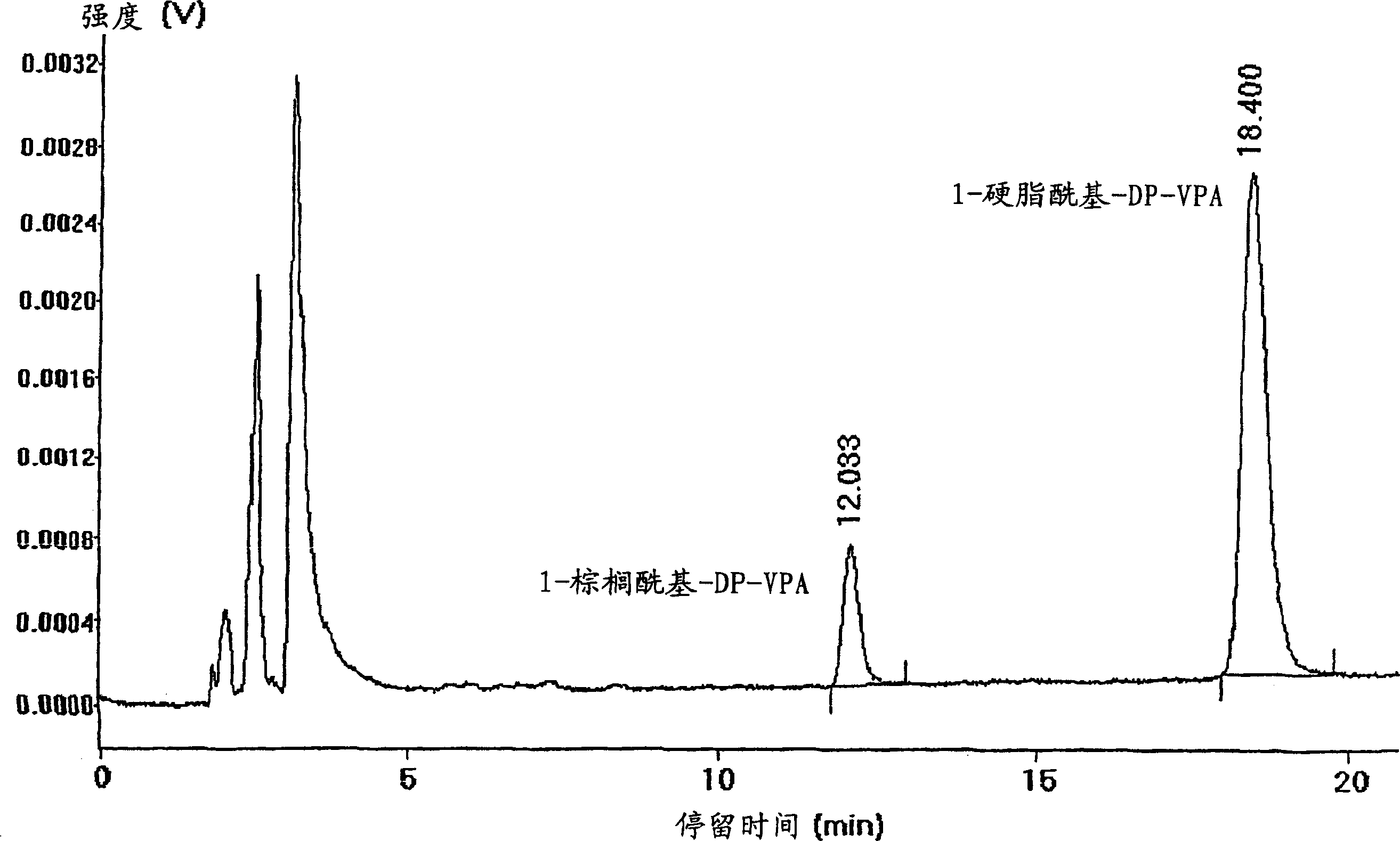
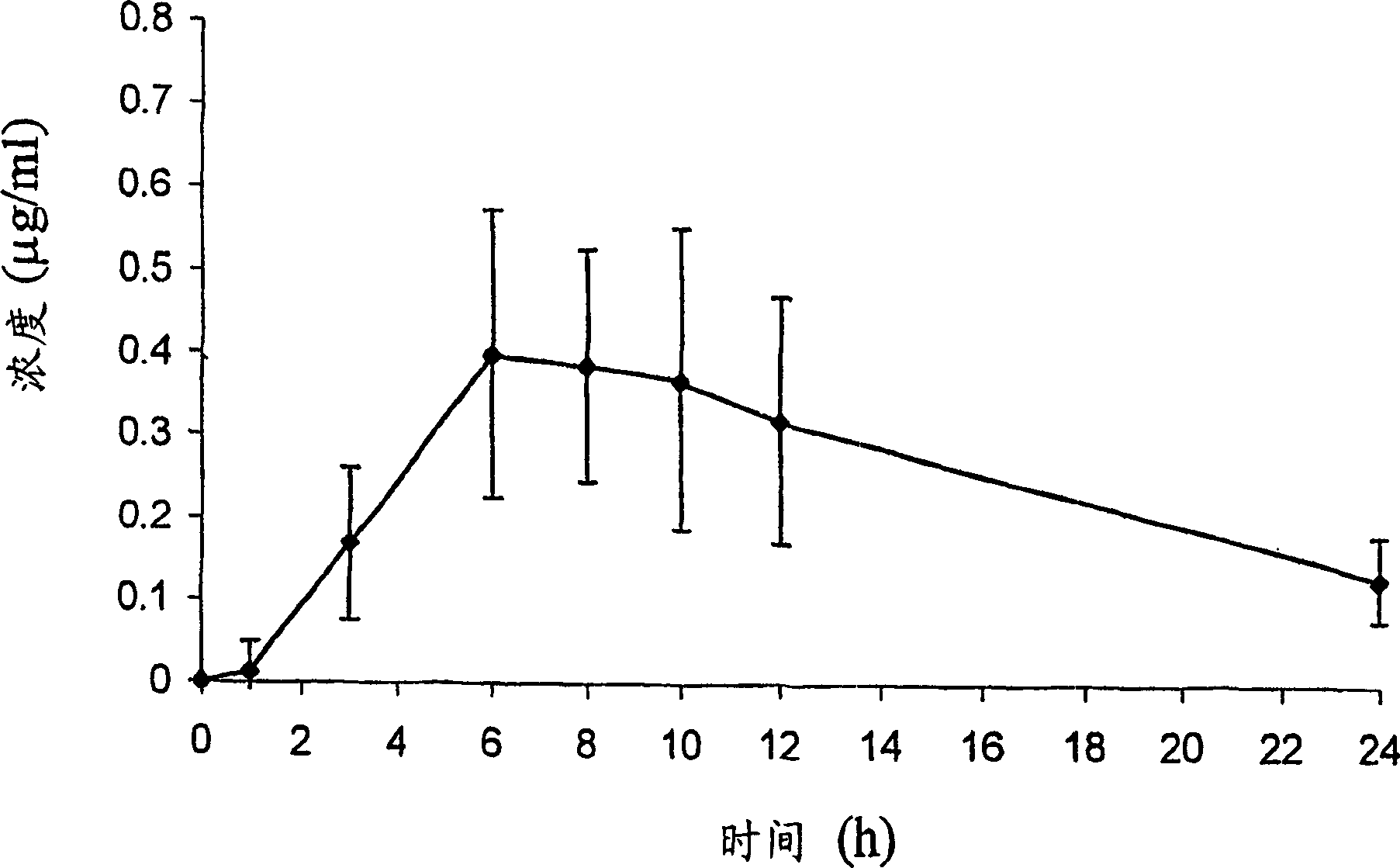
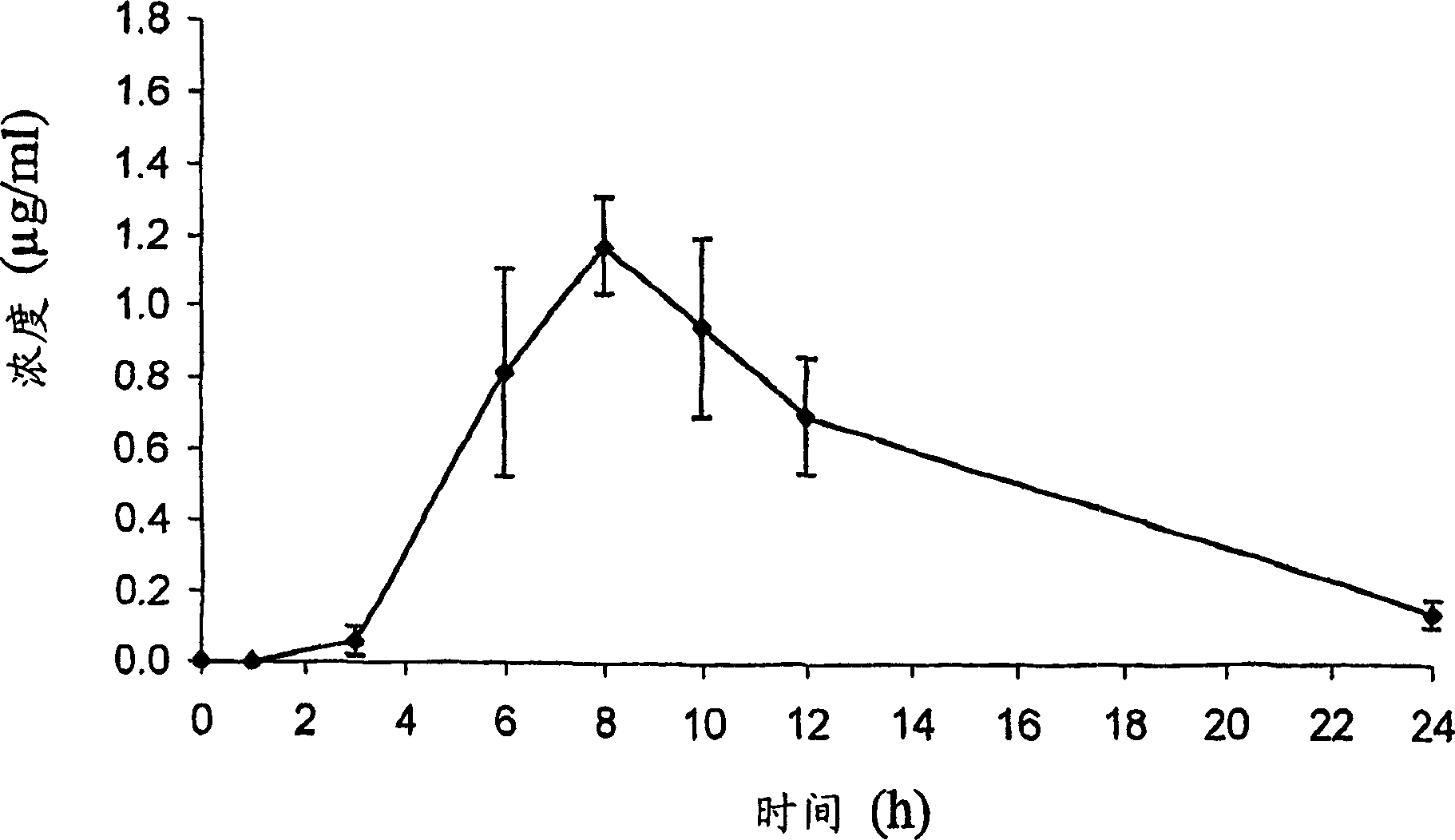
[0065] Example 3: Analysis of DP-VPA Compounds
[0066] For C synthesized in Example 2 as described above 16 / C 18 - The DP-VPA mixture was subjected to analytical assays to identify and demonstrate its structure. 1-palmitoyl-2-valproyl-sn-glyceryl-3-phosphatidylcholine (C 16 -DP-VPA) and 1-stearoyl-2-valproyl-sn-glyceryl-3-phosphatidylcholine (C 18 The analytical results of the product of -DP-VPA) are given below.
[0067] mass spectrometry
[0068] The mass of the protonated DP-VPA molecule determined by ESI(+) is C 16 - 622.4 to 622.8 for DP-VPA; and C 18 -DP-VPA is 650.4 to 650.8. This agrees well with the calculated molecular weight values.
[0069] Elemental analysis
[0070] Press M.H. 2 0 Calculated values: C60.93%; H10.25%; N2.11%; P4.66% (M according to the content).
[0071] The average values determined were C60.72%; H10.58%; N2.09%; P4.56%.
[0072] These values agree well with the calculated values.
[0073] Thin layer chromatography (TLC) analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com