Biologically active native biomatrix composition
A biological matrix and composition technology, applied in the direction of microorganisms, animal/human proteins, biochemical equipment and methods, can solve the problem of increasing artificial results, inability to provide reproducible in vitro BM systems for various physiological and pathological processes, and lack of biological active biomatrix etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
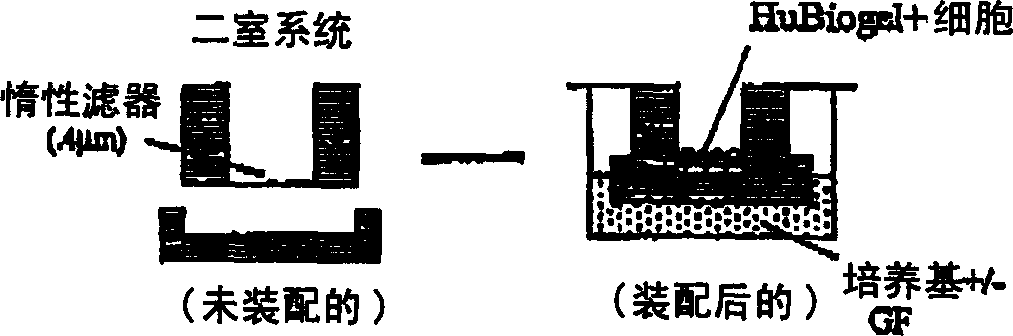
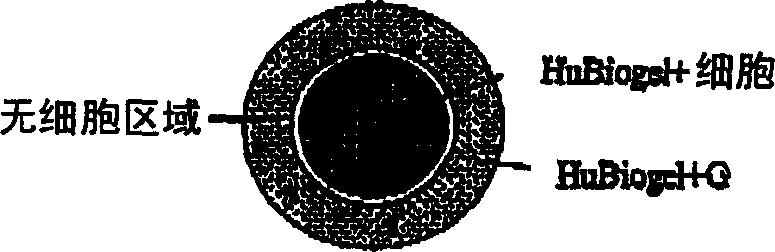
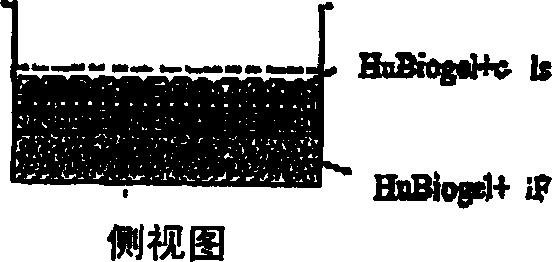
[0024] HuBiogel TM the composition of
[0025] Bioactive biomatrix compositions are disclosed. In one embodiment, the biomatrix is derived from human BM tissue (amnion). In one embodiment, the bioorganic matrix contains laminin, collagen I, collagen IV. In alternative embodiments, the matrix may also contain nestin and tenascin. In yet another alternative embodiment, the biomatrix may contain any combination of nestin, tenascin, fibronectin and proteoglycans, such as heparan sulfate proteoglycans. As used in this disclosure, biological activity refers to the presence of extracellular components necessary to maintain viability and allow growth of various cell types under in vitro culture conditions. In one embodiment, the extract contains (by weight) 16-21% laminin, 35-46% collagen I, 19-33% collagen IV, 7.5-14.5% nestin, 3.5-7% tendon Raw egg whites, 0-2% fibronectin and 0-1.3% proteoglycans. The extract is substantially free of growth factors and proteoglycanases (how...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com