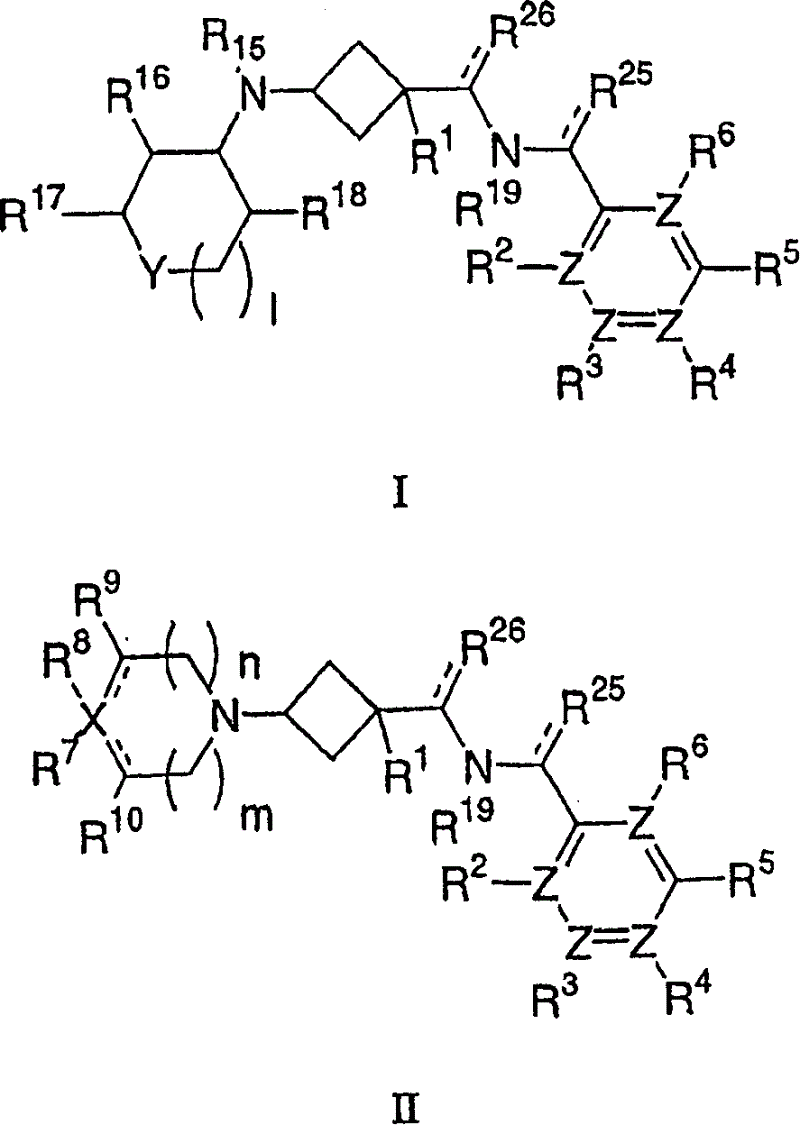
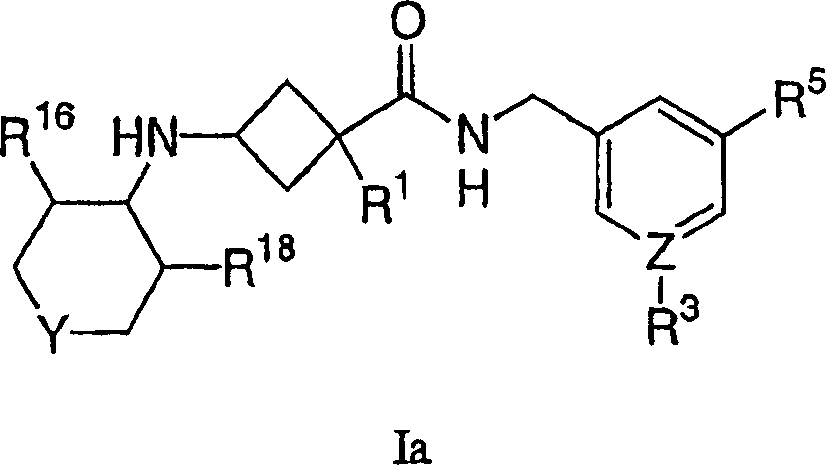
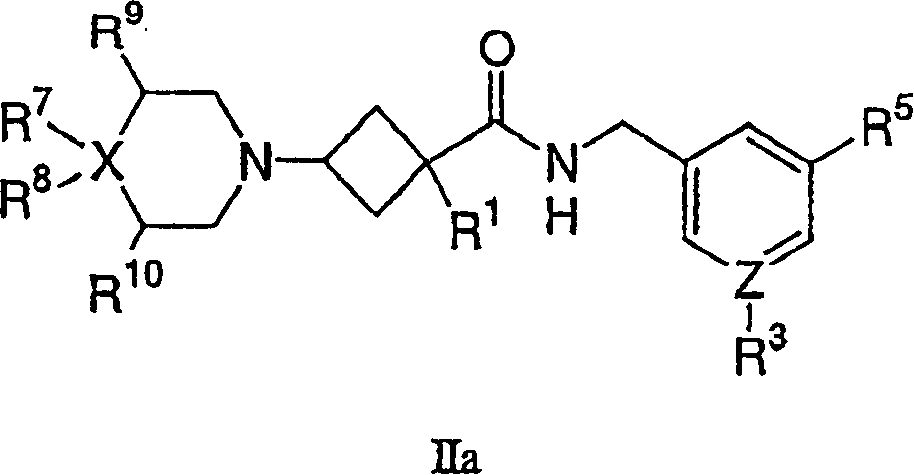
Amino cyclobutylamide modulators of chemokine receptor activity
An alkyl, phenyl technology, applied in the field of aminocyclobutylamide modulators of chemokine receptor activity, can solve the problem of poor sensitivity of rheumatoid arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0641]
[0642] Step A
[0643]
[0644] Intermediate 1 (200 mg, 1.75 mmol), bis(trifluoromethyl)benzylamine (490 mg, 1.75 mmol), DIEA (306 μL, 1.75 mmol), HOAT (240 mg, 1.75 mmol), EDC (504 mg, 2.63 mmol) Combined with DCM (15ml) and stirred at room temperature for 18 hours. The reaction mixture was diluted with DCM, washed with 1N HCl, saturated NaHCO 3 solution, water and brine, washed with anhydrous MgSO 4 Drying and concentration in vacuo afforded the product (529mg, 89%).
[0645] NMR (300MHz, CDCl 3 )δ7.81(s, 1H), 7.74(s, 2H), 6.13(s, 1H), 4.62(d, J=6.04Hz, 2H), 3.56-3.47(m, 2H), 3.29-3.20(m , 2H), 3.07(m, 1H).
[0646] Step B
[0647]
[0648] The product from Step A (100 mg, 0.295 mmol), Intermediate 2 (70 mg, 0.295 mmol), DIEA (103 μL, 0.590 mmol), molecular sieves, NaBH(OAc) 3 (313, 1.474mmol) and DCE (10ml) were mixed together and stirred at room temperature for 18 hours. The reaction was purified by prep...
Embodiment 11
[0655]
[0656] Step A
[0657]
[0658] Intermediate 3 (100 mg, 0.49 mmol), Intermediate 2 (116 mg, 0.49 mmol), DIEA (171 μL, 0.98 mmol), molecular sieves, NaBH(OAc) 3 (520mg, 2.45mmol) and DCM (10ml) were mixed together and stirred at room temperature for 60 hours. The reaction was purified by preparative TLC (2:0.2:97.8, MeOH:NH 4OH:DCM) to give the product (95 mg, 50%). LC-MS for C 26 h 29 NO 2 MW: The calculated value is 387.22, and the measured value is 388.15.
[0659] Step B
[0660]
[0661] The product from step A (90 mg, 0.232 mmol), 5N NaOH solution (325 μL, 1.63 mmol), EtOH (3 ml) and water (2.65 ml) were mixed together and stirred at room temperature. After disappearance of starting material, the reaction mixture was concentrated in vacuo and redissolved in water. The aqueous layer was neutralized to pH 7.0 with 2N HCl solution, then extracted with DCM (7x). The organic layer contained mostly benzyl alcohol. The aqueous layer was concentrated a...
Embodiment 12
[0666]
[0667] Step A
[0668]
[0669] A solution of 60% NaH (10 g, 250 mmol) suspended in DMF (100 ml) was cooled to 0° C., then benzyl cyanide (11.7 g, 100 mmol) was slowly added. The solution was stirred at 0°C for an additional 10 minutes, then dimethoxy-dibromomethane (13.1 g, 50 mmol) was added. The reaction mixture was stirred at 60°C for 18 hours, then cooled to room temperature, poured into water and extracted with ether. The combined organic layers were concentrated in vacuo. The crude product was purified by flash chromatography (20:80, ethyl acetate:hexanes) to give the desired product (6.4 g, 59%).
[0670] NMR (300MHz, CDCl3) δ7.50-7.32(m, 5H), 3.23(d, J=30.6Hz, 6H), 3.11(dm, J=13.6Hz, 2H), 2.73(dm, J=11.7Hz, 2H).
[0671] Step B
[0672]
[0673] A solution of the product from Step A (4.4g, 20mmol), 5M NaOH (20ml), EtOH (50ml) and water (50ml) was heated at reflux overnight then concentrated to dryness. The concentrate was redissolved in water (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com