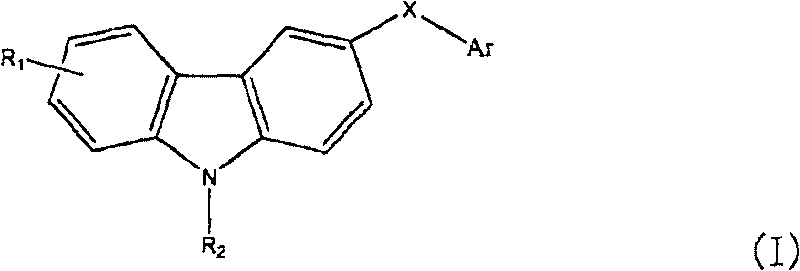
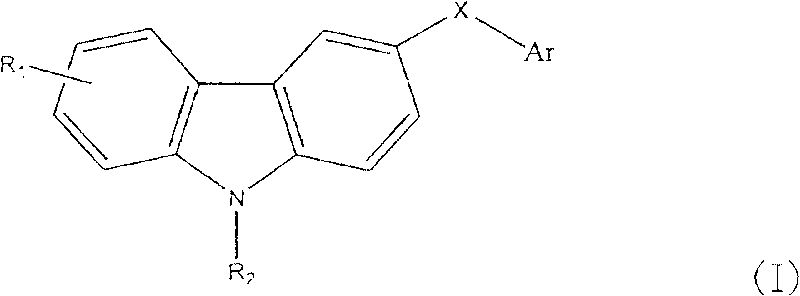
Carbazole sulfonamide derivative and its preparation method
A technology for carbazole sulfonamide and derivatives, which is applied in the field of carbazole sulfonamide derivatives and their preparation, and can solve the problems of difficult synthesis, toxic side effects, poor bioavailability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1: N-(3,4-dimethoxyphenyl)-9-ethylcarbazole-3-sulfonamide (42)
[0133] 9-ethylcarbazole-3-sulfonyl chloride was synthesized according to the method in Mitsumori, Susumu; Tsuri, Tatsuo; Honma, Tsunetoshi et al., Journal of Medicinal Chemistry (2003), 46(12), 2436-2445.
[0134] Add 3mlDMF and 3,4-dimethoxyaniline (80mg, 0.52mmol) to the reaction flask, add homemade 9-ethylcarbazole-3-sulfonyl chloride (150mg, 0.51mmol) while stirring at room temperature, and after 5 minutes , triethylamine (0.11ml, 0.77mmol) was added and the reaction was continued for 2 hours. Then, ice water was added, stirred for a while, the precipitate was filtered, washed with water three times, dried, and separated by VLC to obtain a light brown solid (160 mg, 76%), mp 175-177°C.
[0135] 1 H NMR (DMSO-d 6 ); δ1.30(t, J=7.2Hz, 3H), 3.59(s, 6H), 4.46(q, J=7.2Hz, 2H), 6.56(dd, J=8.7, 2.1Hz, 1H), 6.71 -6.74(m, 2H), 7.27(dd, J=7.5, 7.2Hz, 1H), 7.53(dd, J=7.8, 7.5Hz, 1H), 7.68(d, J=8.1Hz, ...
Embodiment 2
[0139]Example 2: N-(3,5-dimethoxyphenyl)-9-ethylcarbazole-3-sulfonamide (66)
[0140] The product is a light brown solid, yield: 63%; mp 171-173°C.
[0141] 1 H NMR (DMSO-d 6 ); δ1.30(t, J=6.9Hz, 3H), 3.60(s, 6H), 4.46(q, J=6.9Hz, 2H), 6.07(d, J=2.1Hz, 1H), 6.32(d , J=2.1Hz, 2H), 7.28(dd, J=7.5, 7.2Hz, 1H), 7.54(dd, J=7.8, 7.5Hz, 1H), 7.68(d, J=8.1Hz, 1H), 7.77 (d, J=8.7Hz, 1H), 7.85(d, J=8.7Hz, 1H), 8.28(d, J=7.5Hz, 1H), 8.66(s, 1H), 10.20(s, 1H).
[0142] 13 C NMR (DMSO-d 6 );
[0143] Elemental Analysis C 22 h 22 N 2 o 4 S, Calculated: C, 64.37; H, 5.41; N, 6.83. Found: C, 64.70; H, 5.67; N, 6.90.
Embodiment 3
[0144] Example 3: N-(3,4-methylenedioxyphenyl)-9-ethylcarbazole-3-sulfonamide (65)
[0145] The product is a light brown solid, yield: 74%; mp 182-184°C.
[0146] 1 H NMR (DMSO-d 6 ); δ1.30(t, J=6.9Hz, 3H), 4.46(q, J=6.9Hz, 2H), 5.89(s, 2H), 6.50(d, J=8.4Hz, 1H), 6.68-6.71 (m, 2H), 7.27(dd, J=7.5, 7.2Hz, 1H), 7.53(dd, J=7.8, 7.5Hz, 1H), 7.68(d, J=8.4Hz, 1H), 7.72-7.79( m, 2H), 8.25 (d, J = 7.8 Hz, 1H), 8.55 (s, 1H), 9.89 (s, 1H).
[0147] 13 C NMR (DMSO-d 6 );
[0148] Elemental Analysis C 21 h 18 N 2 o 4 S·0.3H 2 O, Calculated: C, 63.07; H, 4.70; N, 7.01. Found: C, 62.99; H, 4.33; N, 6.83.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com