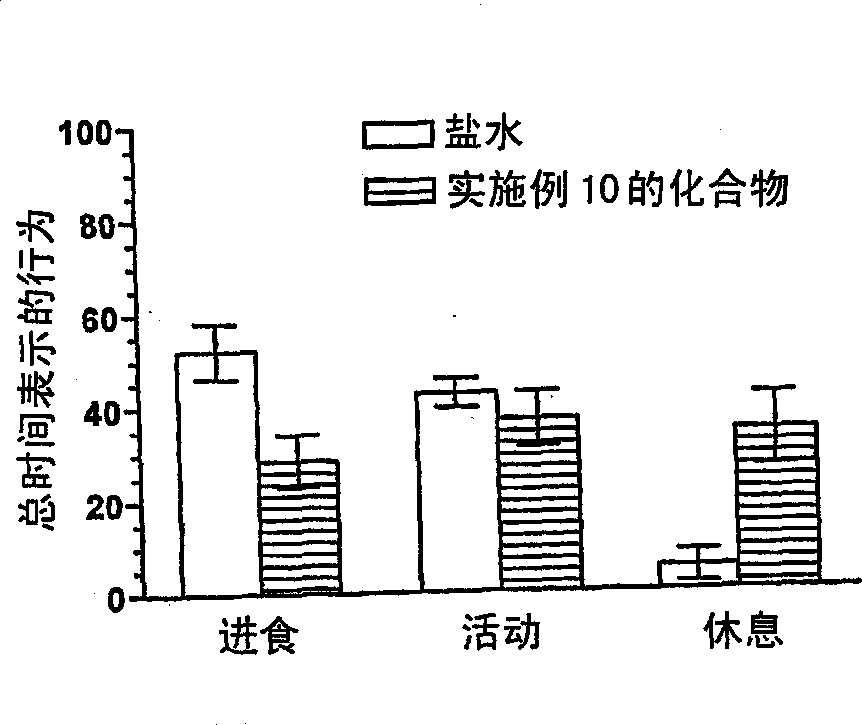
Naphthalene-containing melanocortin receptor-specific small molecule
A compound and stereoisomer technology, applied in the field of 3/061660, can solve the problems of not disclosing the structure of a single substituent, not disclosing the structure of piperazine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 31、32、33 and 34
[0594] The alkyl group on the amino group of the D-4-Cl-phenylalanine moiety in Examples 31, 32, 33 and 34 was introduced by reductive amination as described for the synthesis of mixtures 3-4.
[0595] Route 6: Another Synthesis of Tetra-Substituted Piperazines
[0596]
[0597] To a solution of compound 5-3 and TEA (1 eq) in DCM was slowly added benzyl chloroformate (1 eq) at 0 °C. The reaction was carried out overnight. The product was purified on a chromatographic column after evaporation of the solvent to give 6-1.
[0598] Compound 6-1 was dissolved in anhydrous THF, and borane in THF (1M solution, 5 eq. in total) was added thereto. The solution was stirred for 16 hours. The reaction was quenched with 1N HCl and then the reaction was neutralized with 1N NaOH. The product was extracted with EtOAc and the organic layer was washed with water, brine and dried over sodium sulfate. The solvent was evaporated and the dried product 6-2 was used in the next reaction.
[0...
Embodiment 1
[0642] Example 1 N-3-[1-[2(R)-amino-3-(2,4-dichloro-phenyl)-propionyl]-6(R)-methyl-4-(2-naphthalene -2-yl-ethyl)-piperazin-2(S)-yl]-propyl-guanidine
[0643] The following compounds were obtained by the method of Route 3, using 2-naphthylacetic acid as J-COOH, (S)-(+)-1-amino-2-propanol as NH 2 -CH(R 5 )-CH(R 4)-OH, Fmoc-L-Arg(Boc) 2 -OH as Prt-NH-C(R 2 )-COOH and Boc-D-2,4-dichloro-Phe-OH were synthesized as Q-COOH. Test according to the above method and give the result. Mass spectral analysis: 569.4 (M+H).
[0644]
[0645] Inhibition at 1 μM
[0646] MC1-R MC3-R MC4-R MC5-R
[0647] 14 32 95 36
[0648] Ki(nM)
[0649] MC1-R MC3-R MC4-R MC5-R
[0650] 1309 366 15 727 The compound of example 1 was not intrinsically active at a concentration of 1 μM in the cAMP assay using MC4-R.
Embodiment 2
[0651] Example 2 N-{3-[1-[2(R)-amino-3-(2,4-dichloro-phenyl)-propionyl]-5(R)-methyl-4-(2- Naphthalene-2-yl-ethyl)-piperazin-2(S)-yl]-propyl}-guanidine
[0652] The following compounds are obtained via two routes 3 and 5, using 2-naphthylacetic acid as J-COOH and (R)-(-)-2-amino-1-propanol as NH 2 -CH(R 5 )-CH(R 4 )-OH, Fmoc-LArg(Boc) 2 -OH as Prt-NH-C(R 2 )-COOH, D-alanine methyl ester as NH 2 -CH(R 5 )-COOCH 3 and Boc-D-2,4-dichloro-Phe-OH were synthesized as Q-COOH. Test according to the above method and give the result. Mass spectral analysis: 569.3 (M+H).
[0653]
[0654] Inhibition at 1 μM
[0655] MC1-R MC3-R MC4-R MC5-R
[0656] 20 72 99 65
[0657] Ki(nM)
[0658] MC1-R MC3-R MC4-R MC5-R
[0659] 1134 95 2 362
[0660] In the cAMP assay to determine agonist / antagonist status, compounds other than the ones described were determined to be antagonists of MC4-R.
[0661] At the 1 nmol dose level...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com