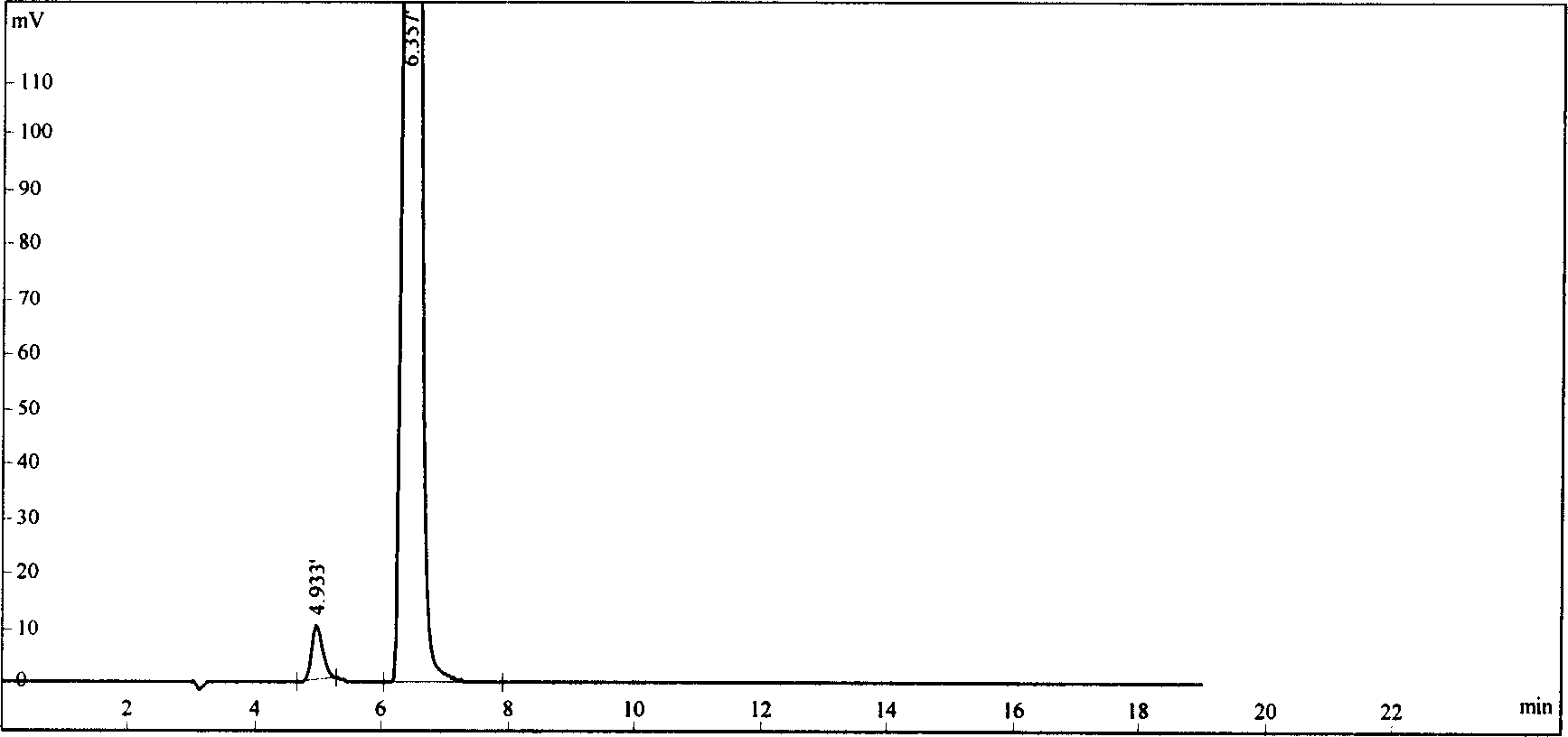
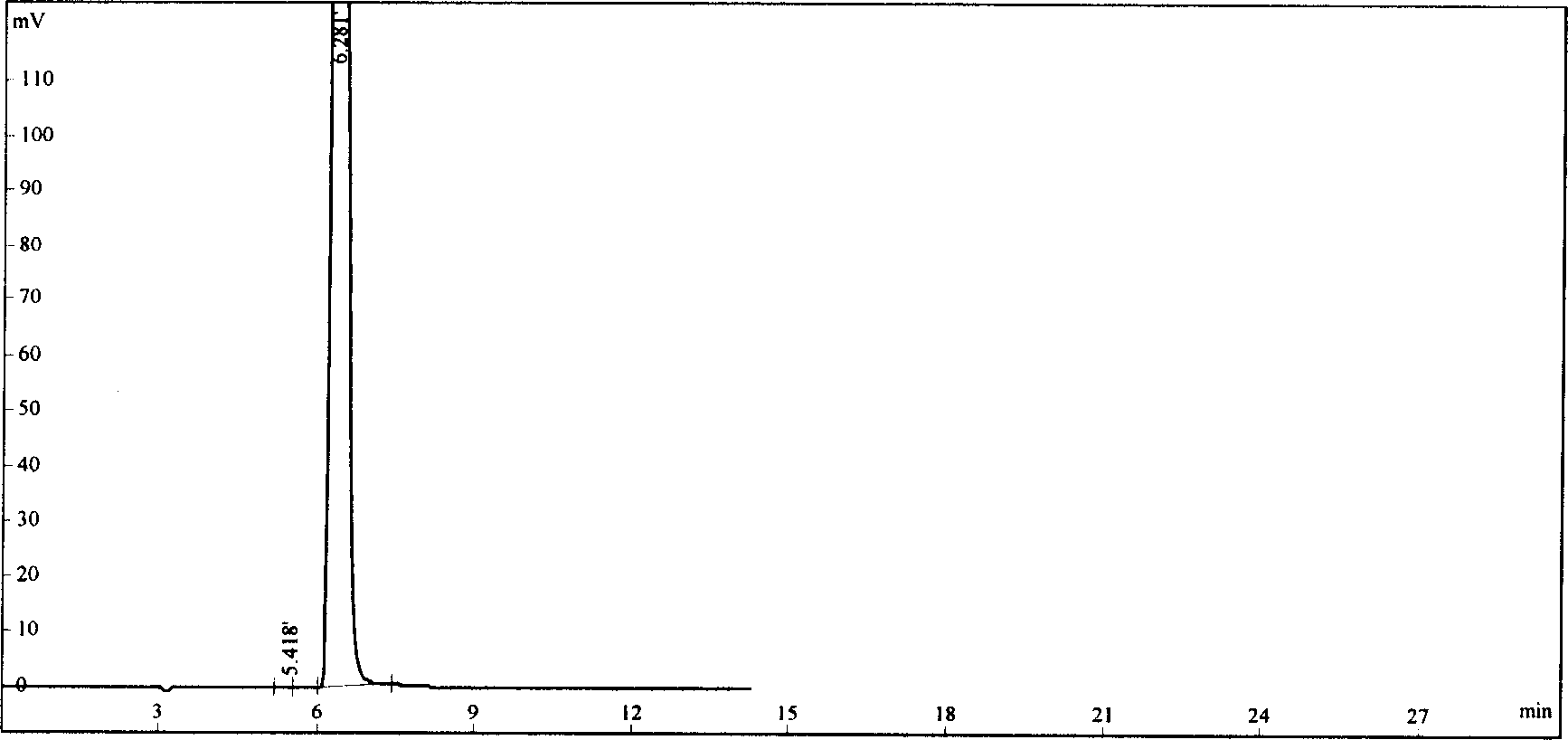
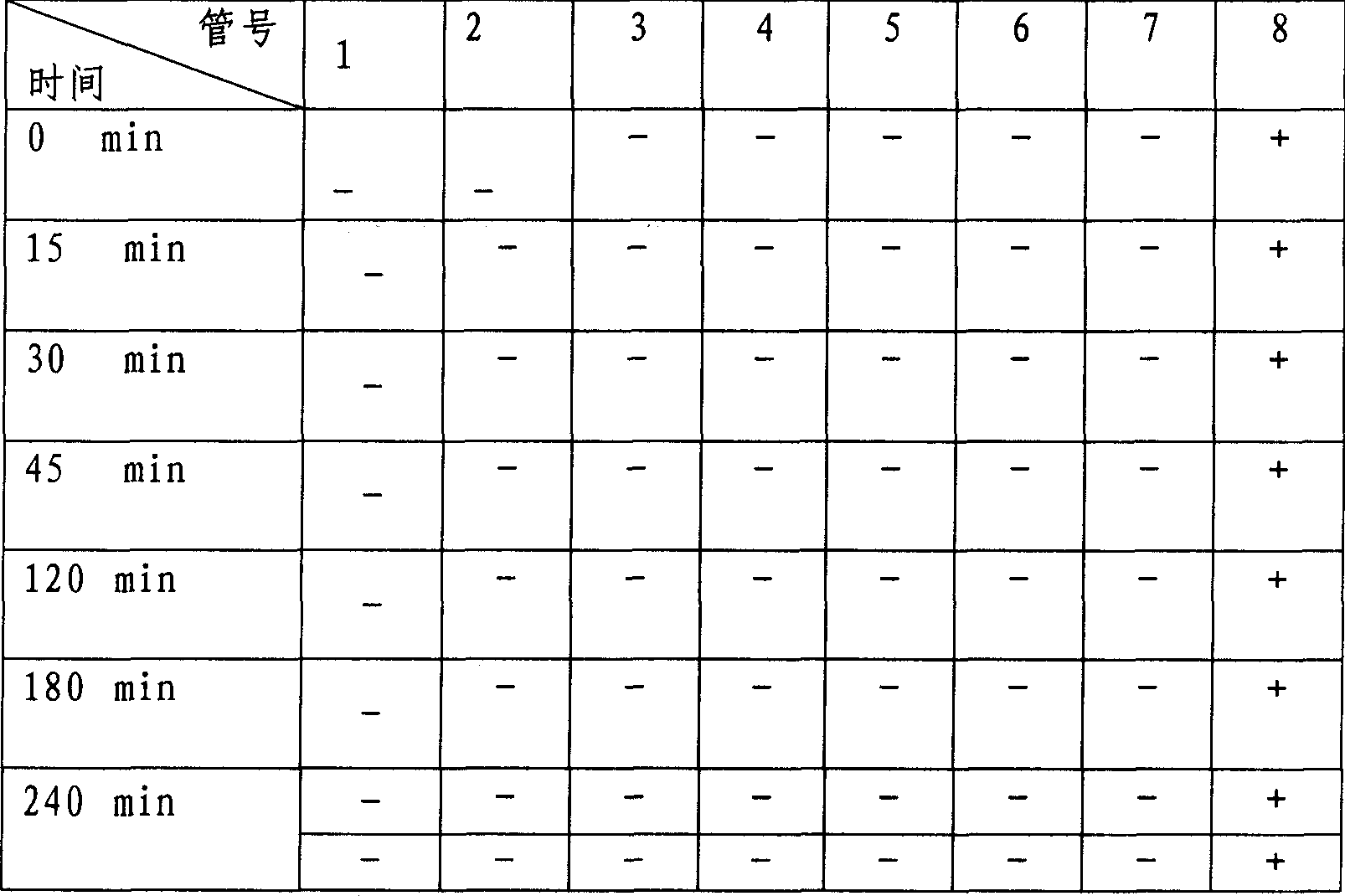
Flunarizine hydrochloride lyophilized agent for injection and its preparing method
A technology of flunarizine hydrochloride and freeze-drying agent, which is applied in the directions of freeze-dried transportation, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of poor stability, inconvenient transportation and inconvenience. It is suitable for long-term storage and other problems, to achieve the effect of stable quality, convenient transportation and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The ratio of raw and auxiliary materials is as follows: (2.5mg / bottle as an example)
[0026] Flunarizine Hydrochloride 9.9g
[0027] Mannitol 323g
[0028] Weigh flunarizine hydrochloride and mannitol according to the above dosage, mix thoroughly and evenly, put in a container, add medicine and water while stirring, adjust the pH to 2.8 with 10% potassium citrate solution, add 10000ml of water for injection, Shake well, add 1g of activated carbon and keep stirring at room temperature for 30 minutes, decarbonize and then filter and sterilize through a 0.22um microporous membrane, pack in aliquots, 3ml per bottle, use quick-freezing method, cool down to -40°C, and keep for 4 hours , evacuated, and freeze-dried. The first stage of sublimation, the temperature should be controlled at -20°C. The vacuum degree was controlled at 0.1 mbar for 30 hours. The second stage of sublimation. The temperature is 40°C, and the time is 4 hours. After freeze-drying, cover and seal. ...
Embodiment 2
[0030] The ratio of raw and auxiliary materials is as follows: (5mg / bottle as an example)
[0031] Flunarizine Hydrochloride 12.9g
[0032] Mannitol 390g
[0033] Weigh flunarizine hydrochloride and mannitol according to the above dosage, mix thoroughly and evenly, put in a container, add medicine and water while stirring, adjust the pH to 3.0 with 10% sodium citrate solution, add 10000ml of water for injection, Shake well, add 10g of needles and stir with activated carbon at room temperature for 30 minutes, decarbonize and then filter and sterilize through a 0.22um microporous membrane, pack in aliquots, 4.5ml per bottle, use quick-freezing method, cool to -50°C, and maintain for 6 hours , evacuated and freeze-dried. In the first stage of sublimation, the temperature should be controlled at -20°C. The vacuum was controlled at 0.1 mbar for 32 hours. The second stage of sublimation. The temperature is 40°C, and the time is 6 hours. After freeze-drying, cover and seal.
Embodiment 3
[0035] The ratio of raw and auxiliary materials is as follows: (2.5mg / bottle as an example)
[0036] Flunarizine Hydrochloride 9.9g
[0037] Mannitol 160g
[0038] Weigh flunarizine hydrochloride and mannitol according to the above dosage, mix well, put in a container, add medicine and water while stirring, adjust pH to 3.5 with 10% sodium hydroxide solution, add water for injection to 10000ml, Shake well, add 5g of activated carbon and keep stirring at room temperature for 30 minutes, decarbonize and then pass through a 0.22um microporous membrane to filter and sterilize, subpackage, 3ml per bottle, use quick-freezing method, cool down to -40°C, and maintain for 6 hours , evacuated, and freeze-dried. The first stage of sublimation, the temperature should be controlled at -20°C. The vacuum was controlled at 0.1 mbar for 28 hours. The second stage of sublimation. The temperature is 40°C, and the time is 3 hours. After freeze-drying, cover and seal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com