Production of recombinant insulinum primary C peptide
A technology for human insulin and nucleotide sequences, which is applied in the field of efficient production of recombinant human proinsulin C-peptide, expression and purification of recombinant human proinsulin C-peptide, and can solve problems such as difficult detection of short peptide expression products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The acquisition of embodiment 1 target gene and the construction of expression plasmid
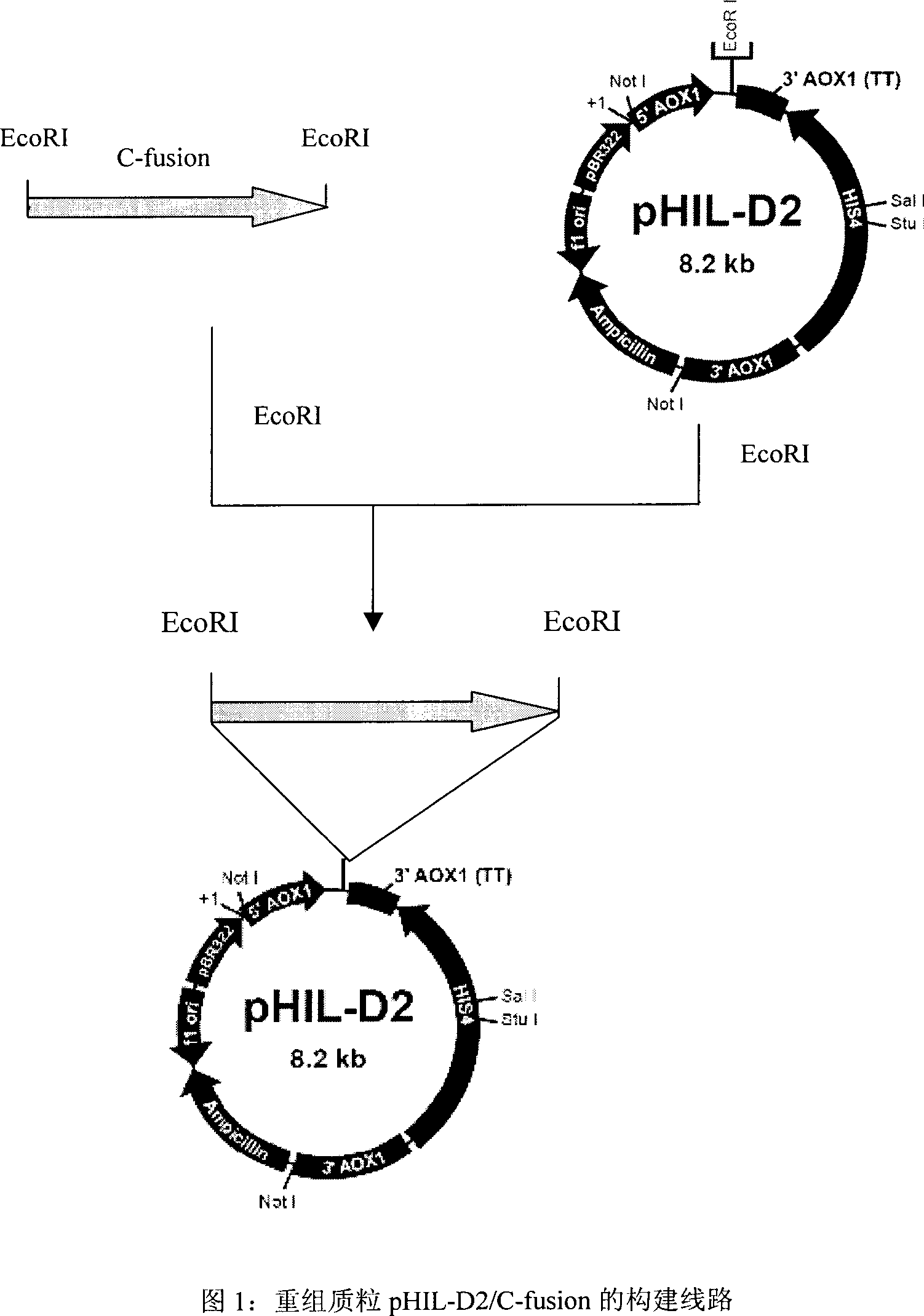
[0054] Since the human proinsulin C-peptide has only 31 amino acids, its molecular weight is too small, and in order to increase the yield of the final product, we intend to express multiple C-peptide genes in series. According to the amino acid sequence of human proinsulin C-peptide and the codon bias of Pichia pastoris, and in order to facilitate the insertion of the tandem C-peptide fusion protein gene into the expression vector and further purification after expression, we introduced EcoRI sites at both ends of the tandem C-peptide gene point, and introduced six His at its 5' end and a stop codon at its 3' end. We designed and synthesized a human proinsulin C-peptide fusion protein gene suitable for expression in Pichia pastoris. Its sequence is shown in SEQ ID NO: 1, which contains eight tandem C-peptide genes in the correct direction.
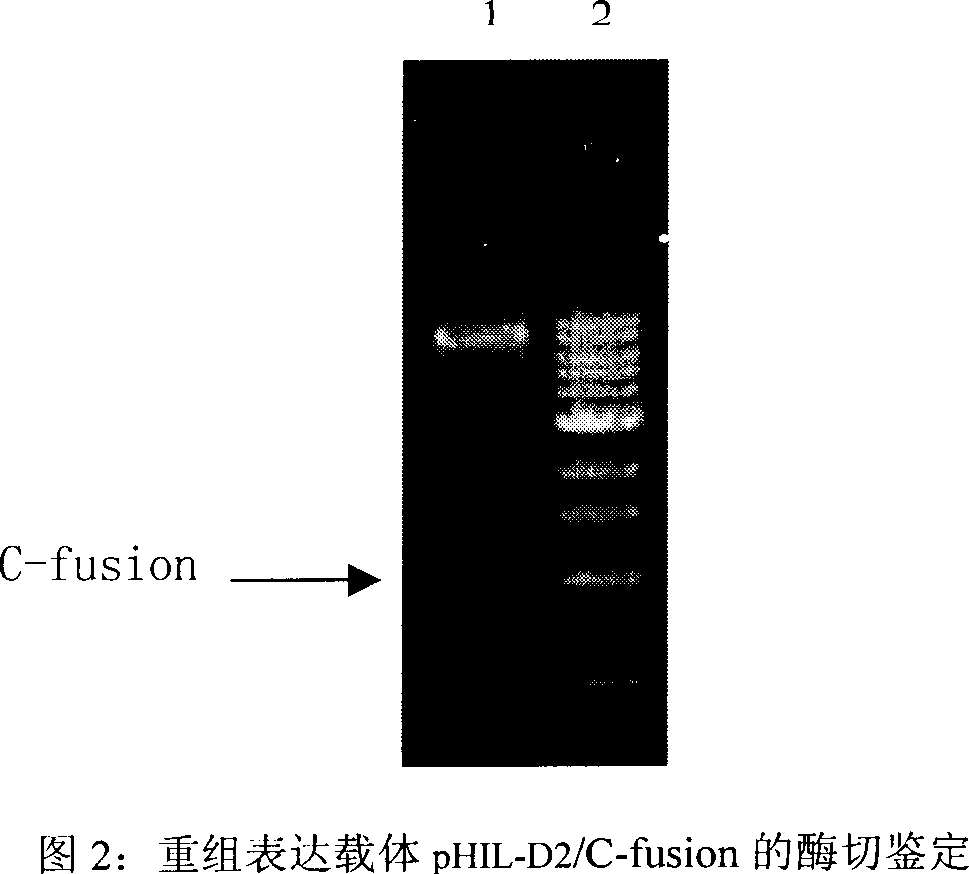
[0055] The recombinant plasmid construc...
Embodiment 2
[0056] Example 2 Screening of Highly Expressed C Peptide Engineering Strains
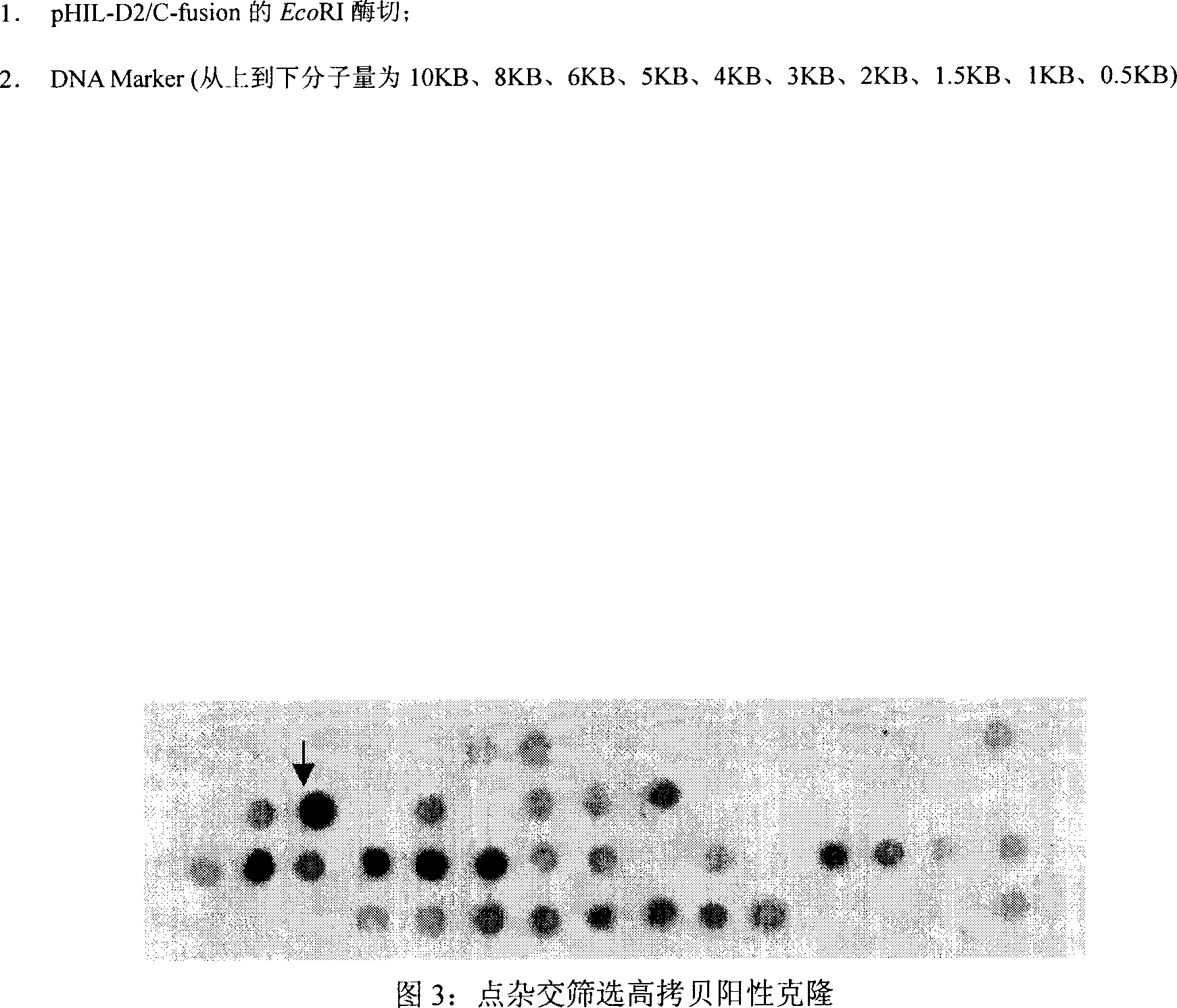
[0057] A large amount of the constructed recombinant plasmid pHIL-D2 / C-fusion was prepared, linearized, electroporated and transformed into host cell P. pastoris GS115, and positive clones were screened on the MD plate. Since the MD plate does not contain histidine, only the recombinant plasmid was transfected. Positive clones can only grow after entry. Extract the total DNA of positive clones, use C-fusion gene as a probe, and screen high-copy positive clones by dot hybridization. As shown in Figure 3, the darker the color, the more C-fusion genes contained in the genome, that is, the higher the copy number.
[0058] Select several high-copy positive clones, inoculate them into BMGY medium, grow to saturation state, replace the medium BMMY induced expression, take cell lysate after 48 hours, after preliminary purification, SDS-PAGE detection, obtain a high Expression cloning.
Embodiment 3
[0059] The influence of embodiment 3pH on expression level
[0060] Take a single clone, inoculate it into the BMGY primary seed solution, and cultivate it for 17-20hr; secondly inoculate it in a 500ml Erlenmeyer flask with 50ml medium at a ratio of 1:10 (each condition has an auxiliary tube), and cultivate it for about 24hr, 1 % Methanol induction, the pH of the induction stage needs to be adjusted according to the table below (directly adjust the pH in BMMY medium). Sampling was taken at 24 hours after induction, OD and pH were measured at the same time, and 1% methanol was added to continue induction. A total of 48hr induction. SDS-PAGE and immunoblotting were used to determine the content of tandem C-peptides before induction and at different times of induction.
[0061] The experimental results showed that the optimum pH for expressing C-peptide was between 5 and 7 at the shake flask level. Optimum pH6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com