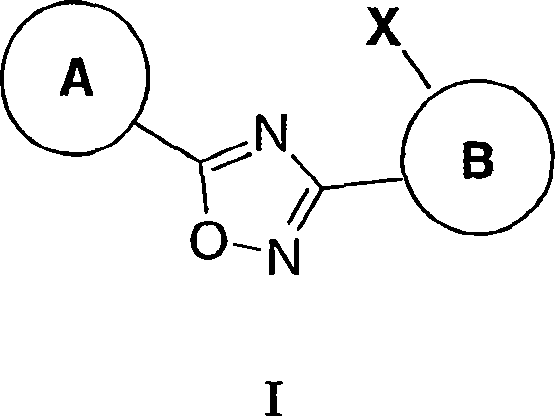
3,5-aryl, heteroaryl or cycloalkyl substituted-1,2,4-oxadiazoles as S1P receptor agonists
A cycloalkyl compound technology, applied in the field of 3,5-aryl, heteroaryl or cycloalkyl-substituted 1,2,4-oxadiazole compounds as S1P receptor agonists, capable of Work around limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267] 3-(2-Methyl-5-chlorophenyl)-5-(4-(2-methylpropyl)phenyl)-1,2,4-oxadiazole
[0268] Step A: N-Hydroxy-(2-methyl-5-chloro)benzamidine
[0269] A mixture of 2.50 g (16.5 mmol) 5-chloro-2-methylbenzonitrile, 2.30 g (33 mmol) hydroxylamine hydrochloride and 6.90 g (82.5 mmol) sodium bicarbonate in 25 mL MeOH methanol was stirred at 50° C. for 16 h. The reaction mixture was cooled, diluted with 50 mL 2N HCl, then washed with 3 x 30 mL CH 2 Cl 2 and 1 x 30 mL EtOAc for extraction. The combined organics were dried and concentrated to give 2.15 g of the title compound:
[0270] 1 H NMR (500MHz, CD 3 OD): δ7.29-7.34 (m, 2H), 7.23 (d, J=8.0, 1H), 2.38 (s, 3H). Step B: 3-(2-methyl-5-chlorophenyl)- 5-(4-(2-methylpropyl)phenyl)-1,2,4-oxadiazole
[0271] 500mg (2.8mmol) 4-(2-methylpropyl) benzoic acid, 600mg (3.1mmol) hydrochloride 1-(3-(dimethylamino)propyl)-3-ethylcarbodiimide and 420mg A mixture of (3.1 mmol) 1-hydroxybenzotriazole in 10 mL of acetonitrile was stirred at rt...
Embodiment 2-18
[0274] The following compounds were prepared using methods similar to those described in Example 1, substituting the appropriate carboxylic acid for 4-(2-methylpropyl)benzoic acid in Step B.
[0275]
[0276]
[0277]
[0278]
[0279]
Embodiment 19-25
[0281] The following compounds were prepared in a manner similar to those described in Example 1, substituting the appropriate nitrile for (2-methyl-5-chloro)benzonitrile in Step A and 4-(cyclohexyl) Benzoic acid was substituted for 4-(2-methylpropyl)benzoic acid.
[0282]
[0283]
[0284]
[0285]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com