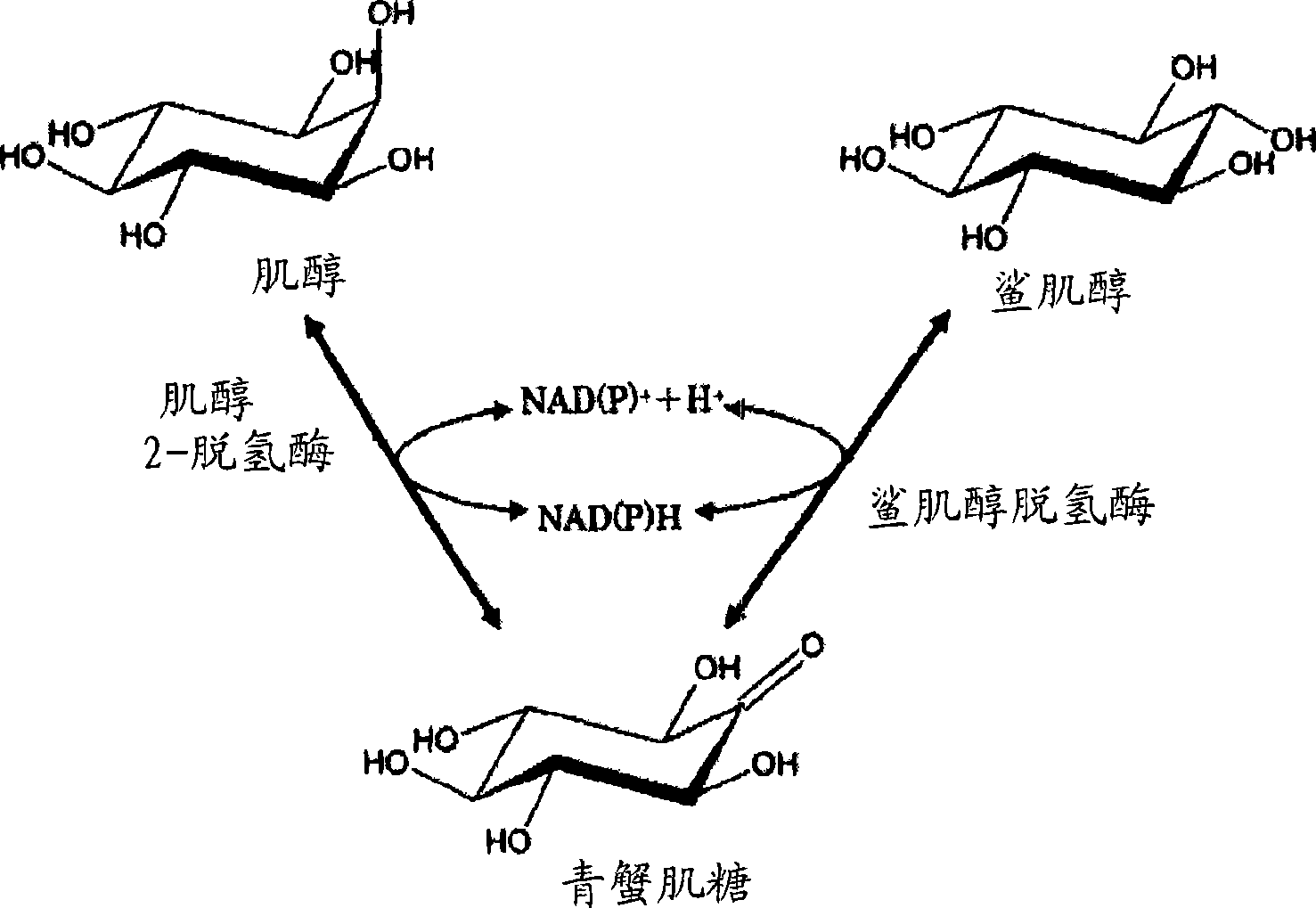
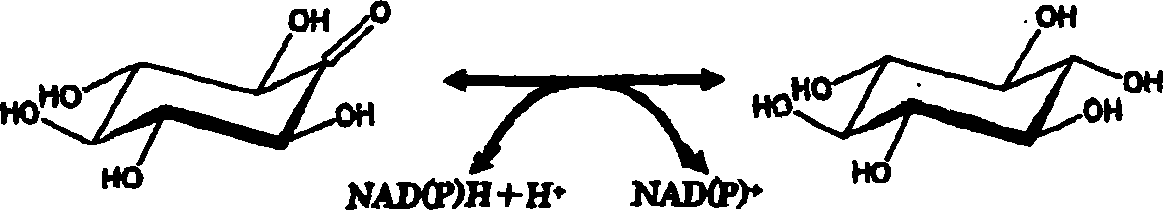
Process for producing scyllo-inositol
A technology of scyllo-inositol and inositol, applied in the field of preparation of scyllo-inositol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0342]
[0343] 3 liters of liquid medium containing 10.0% inositol, 1.0% yeast extract, and 1.0% sucrose were adjusted to pH 7.0 using 1N NaOH, and 100 ml were injected into 30 Erlenmeyer flasks with baffles each with a volume of 500 ml. Sterilize by autoclave. Each Erlenmeyer flask was inoculated with a slant culture 1 loop of Acetobacter AB10281FERM BP-10119, and cultured on a shaker at 27° C. for 5 days. After the cultivation, 250 ml of water was added to each Erlenmeyer flask, and the mixture was stirred for 1 hour on a shaker to dissolve the crystalline scyllo-inositol present in the culture solution. The culture solution was collected, centrifuged (8,000 rpm for 20 min), and the supernatant was obtained as the culture solution supernatant (10.2 L).
[0344] The culture supernatant was analyzed by high-speed liquid chromatography under the following conditions. As a result, 12.6 mg / ml scyllo-inositol (129 g, conversion rate 43%) was produced in the culture supernatan...
Embodiment 2
[0360]
[0361] A 40-liter liquid medium containing 10.0% inositol, 1.0% yeast extract, and 1.0% sucrose was introduced into a 50-liter fermenter, adjusted to pH 7.0 with 1N NaOH, and sterilized in an autoclave. Next, 400 ml of Acetobacter AB10281FERM BP-10119 cultured in a culture medium (erlenmeyer flask) with the same composition was cultured at 27° C. for 5 days at an aeration rate of 1 vvm and a rotational speed of 200 rpm. After culturing, 60 L of warm water at about 50° C. was added to about 40 L of culture solution taken out, and stirred for 1 hour to dissolve crystalline scyllo-inositol present in the culture solution. This culture solution was continuously centrifuged (8,000 rpm) to obtain a solution from which bacterial cells were removed as a culture sterilizing solution (about 100 L).
[0362] This culture sterilized solution was analyzed by high-speed liquid chromatography under the following conditions. As a result, 16.8 mg / ml scyllo-inositol (1.68 kg, conver...
Embodiment 3
[0367]
[0368] The 16S rRNA base sequences of four strains including three naturally isolated strains AB10285, AB10286, and AB10287, which have the ability to convert inositol into scyllo-inositol, and AB10281 bred from AB10253 were analyzed according to conventional methods. That is, after the genomic DNA was extracted from the cultured thalline, the corresponding DNA fragment was amplified by PCR with primers designed to amplify about 1.6kbp of 16SrRNA, and then the sequence related to about 1.3kbp was analyzed (Hokkaido System Science Corporation ). Based on the sequence results and compared with the database, related species were identified.
[0369] Table 2 shows the control results, homology, and the conversion rate to scyllo-inositol when cultured in the same manner as in Example 1.
[0370] system name
[0371] From this result, it was revealed that the four strains having the ability to convert inositol into scyllo-inositol can be roughly divided into ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear gradient | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com