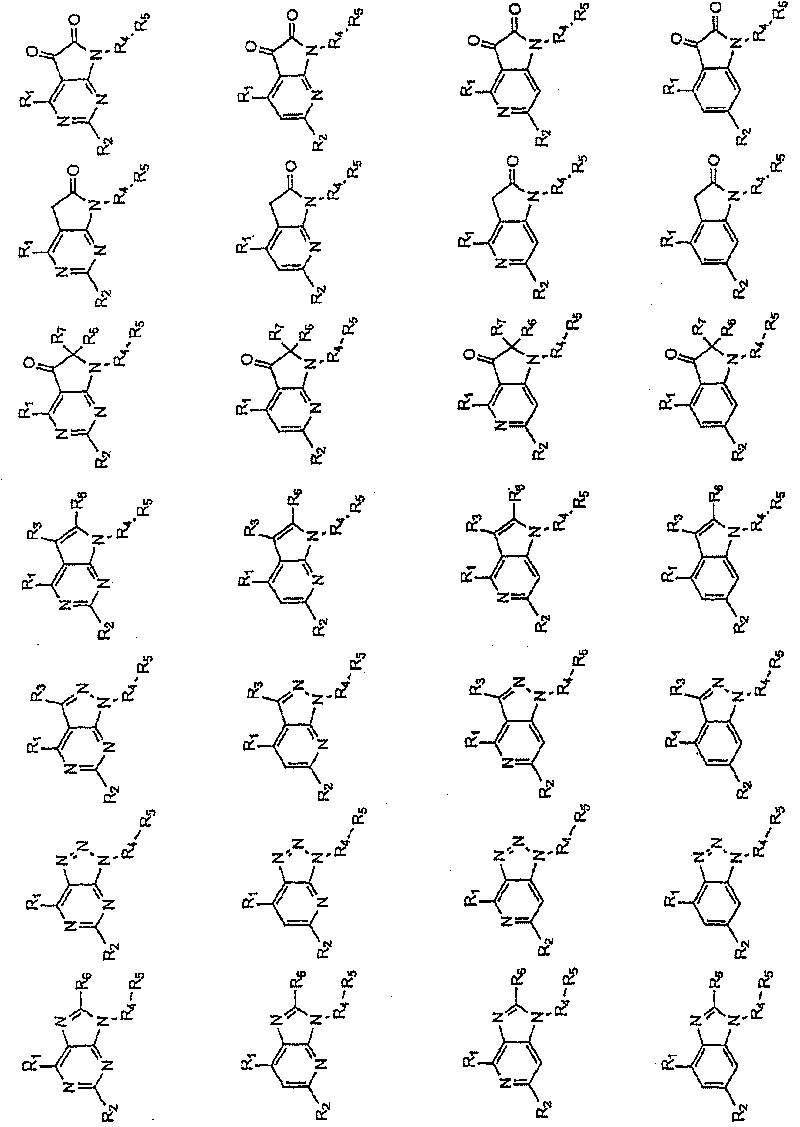
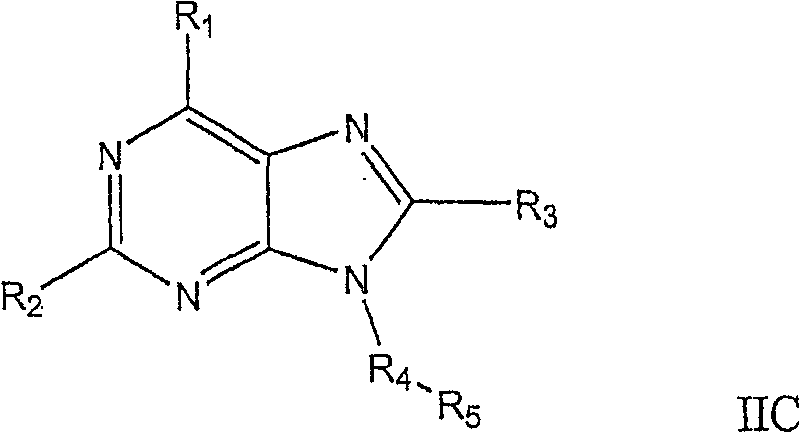
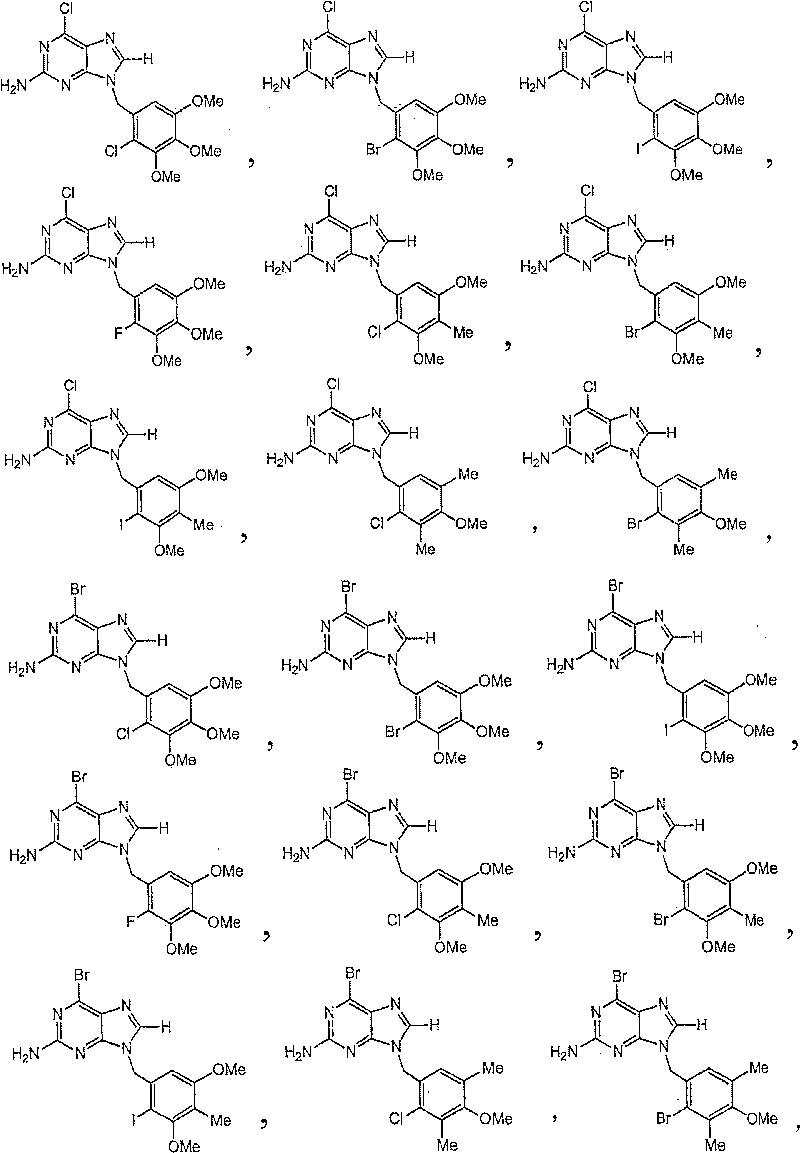
Novel heterocyclic compounds as HSP90-inhibitors
A kind of compound, the technology of heterocyclic group, be applied in the field of heterocyclic compound, can solve the problem such as not being reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1221] Example 1. 2-Chloro-1-chloromethyl-3,4,5-trimethoxy-benzene
[1222]
[1223] According to the general procedure 3.1, 5-chloromethyl-1,2,3-trimethoxy-benzene was chlorinated with NCS to obtain the title compound.
[1224] 1 H-NMR(CDCl 3 ): δ 6.82 (s, 1H), 4.70 (s, 1H), 3.93 (s, 3H), 3.90 (s, 3H) 3.87 (s, 3H).
Embodiment 2
[1225] Example 2. 2-Chloro-6-chloromethyl-4-methoxy-3,5-dimethyl-pyridine
[1226]
[1227] Step 1: 2-Chloromethyl-4-methoxy-3,5-dimethylpyridine-1-oxide
[1228] Follow general procedure 2.1 to oxidize 2-chloromethyl-4-methoxy-3,5-dimethyl-pyridine to obtain the title compound. HPLC Rt: 4.46 minutes. 1 H-NMR(CDCl 3 ): δ 8.05 (s, 1H), 4.93 (s, 2H), 3.77 (s, 3H), 2.37 (s, 3H), 2.24 (s, 3H).
[1229] Step 2: 2-Chloro-6-chloromethyl-4-methoxy-3,5-lutidine
[1230] Follow the general step 2.6 with POCl 3 Treatment of 2-chloromethyl-4-methoxy-3,5-lutidine-1-oxide gave the title compound. HPLC Rt: 6.757 minutes. 1 H-NMR(CDCl 3 ): δ 4.64 (s, 2H), 3.79 (s, 3H), 2.35 (s, 3H), 2.33 (s, 3H).
Embodiment 3
[1231] Example 3. 4-Chloro-2-chloromethyl-3,5-dimethyl-pyridine
[1232]
[1233] Use POCl in the same way as in the general step 2.6 3 Treatment of 2-chloromethyl-3,5-lutidine-4-ol (Tarbit, et al., WO 99 / 10326) gave the title compound (74% yield). HPLC Rt: 5.54 minutes. 1 H-NMR(CDCl 3 ): δ 8.24 (s, 1H), 4.71 (s, 2H), 2.48 (s, 3H), 2.36 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com