Methods and products which utilize N-acyl-L-aspartic acid
一种烷基、芳基的技术,应用在利用N-酰基-L-天冬氨酸和产品领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0028] A. Compound
[0029] The present invention provides methods and products using a compound of formula I or a pharmaceutically acceptable salt thereof:
[0030] R 1 -C(O)-NH-CH 2 (CH 2 -COOR 2 )-COOR 2 (I)
[0031] among them
[0032] R 1 Is H, lower alkyl or lower alkyl substituted by halogen atoms, and
[0033] R 2 , Each R 2 They may be the same or different and are H or alkyl, cycloalkyl, aryl, alkaryl or aralkyl, each of which may be optionally substituted with a polar substituent.
[0034] Highly preferred is N-acetyl-L-aspartic acid (NAA) or a pharmaceutically acceptable salt of NAA.
[0035] "Alkyl" as used herein means a linear or branched saturated hydrocarbon, preferably containing 1-30 carbon atoms, more preferably containing 1-20 carbon atoms (e.g., methyl, ethyl, propyl, isopropyl Wait).
[0036]As used herein, "aryl" means an aryl group containing at least one aromatic ring (e.g., phenyl).
[0037] As used herein, "alkaryl" means an alkyl group having an ary...
Embodiment 1
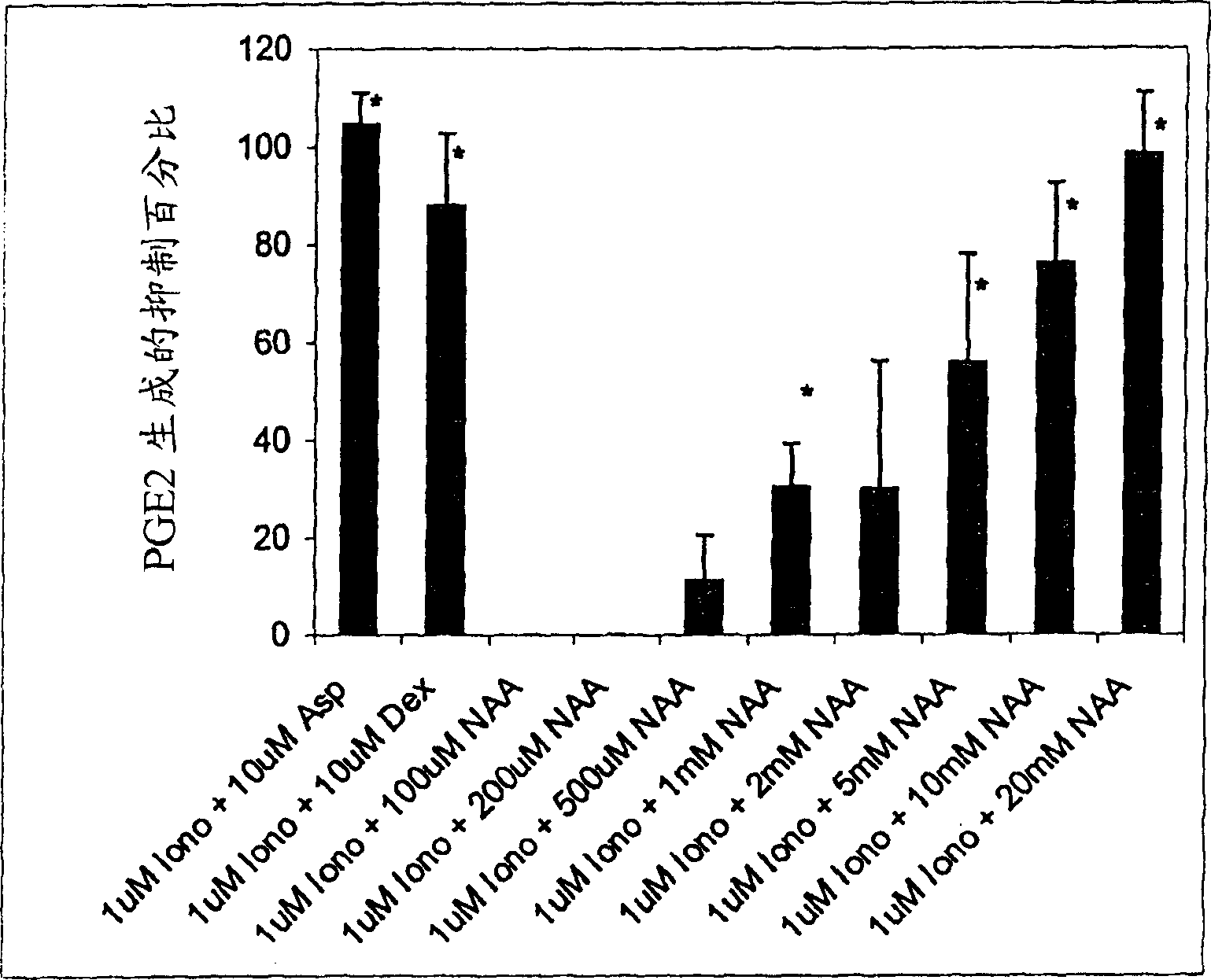
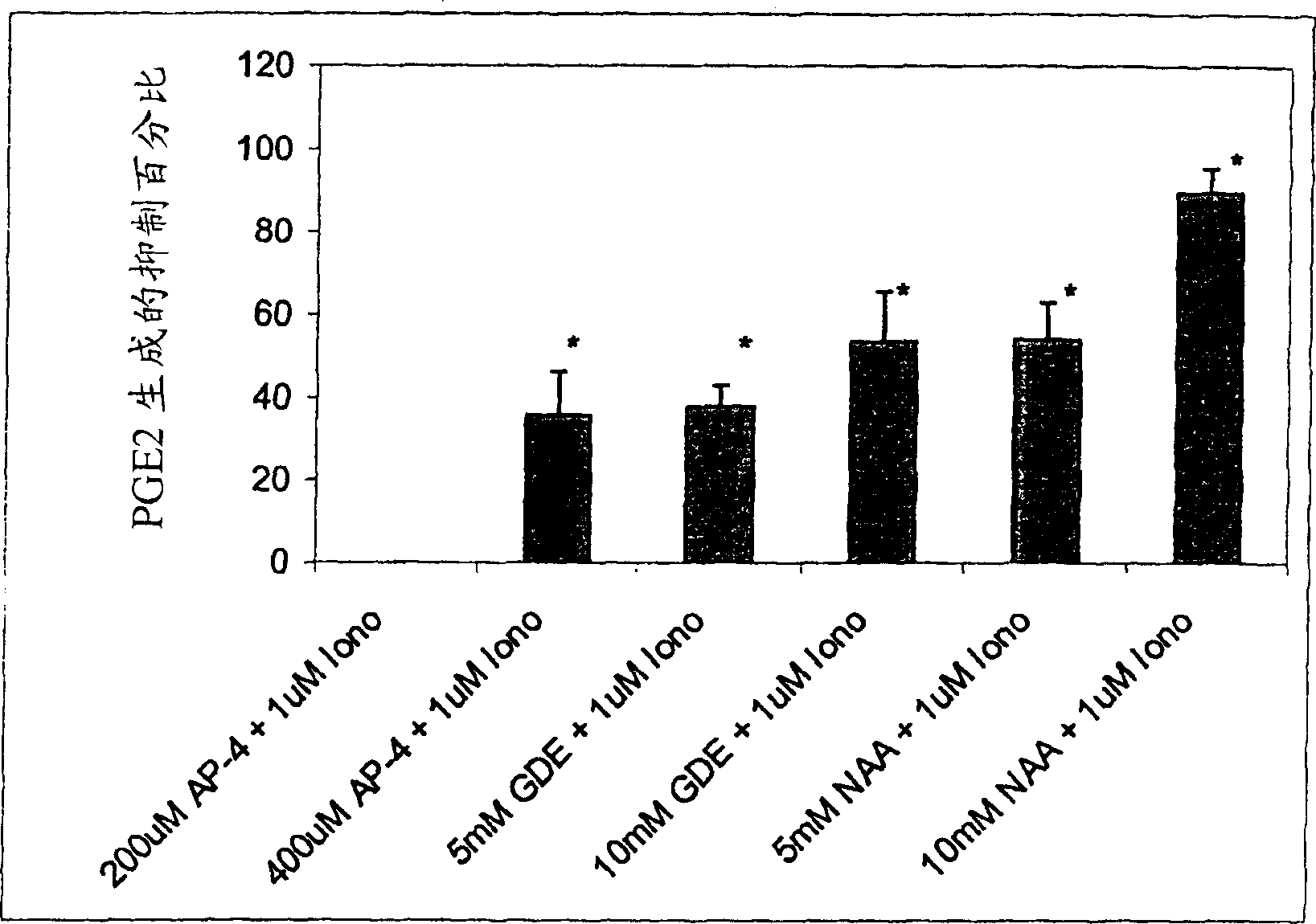
[0188] Although N-acetyl-aspartic acid (NAA) has been shown to play an important role in myelin synthesis and osmotic pressure regulation, the biological principle of high levels of NAA in the brain is still unknown. In this example, a human astrocyte cell line (STTG) was treated with NAA and stimulated with Ionomycin or IL-1β. By measuring inflammatory mediators such as prostaglandin E 2 (PGE 2 ), cyclooxygenase-2 (COX-2) protein and activated NFκB to study the secondary inflammatory response. When NAA concentration is 10 and 20mM, PGE in STTG cells stimulated by Ionomycin 2The levels have dropped by 76% and >95%, respectively. Glutamate receptor antagonists (L-AP-4 and L-glutamate diethyl ester) also caused a decrease in PGE2 levels in STTG cell lines. NAA also reduced the amount of COX-2 protein and activated NFκB in STTG cells stimulated by IL-1β, but had little effect on unstimulated cells. NAA has no effect on overall COX-2 activity or COX-2 mRNA. These results demonstrate t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com