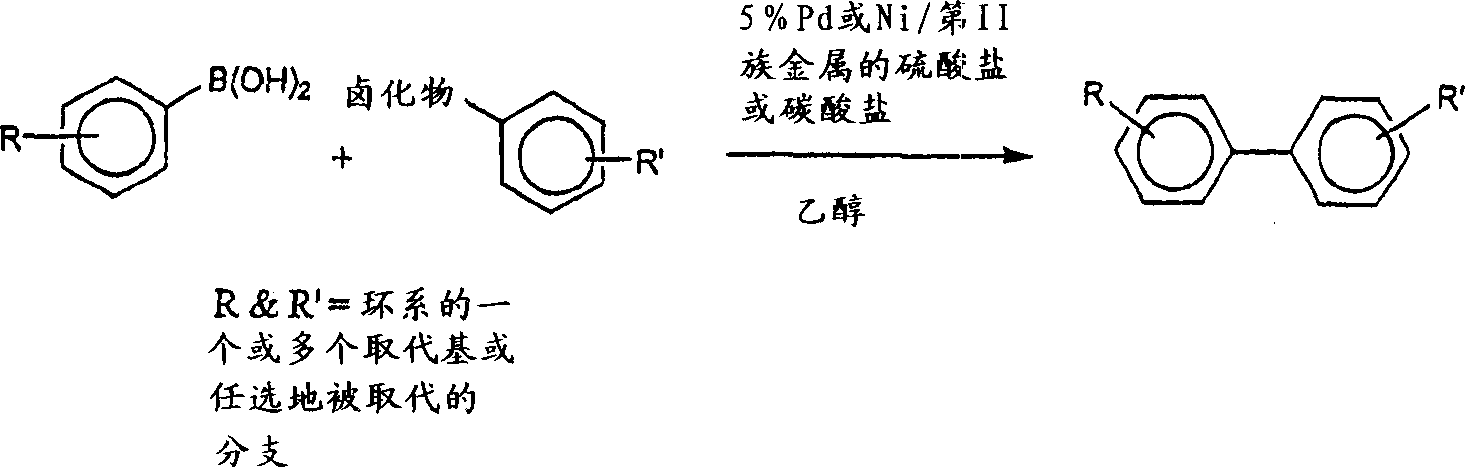
Carbon-carbon cross coupling catalyzed by transition metals on solid supports
一种固体载体、偶联的技术,应用在制备二芳基化合物,偶联芳基化合物领域,能够解决难以使用、去除炭载体困难、不能大批量使用等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1-catalyst in the preparation of 3-chloro-5-(4,4-dimethyl-2-oxo-1,4-dihydro-2H-benzo[b][1,3]oxazine-6 -base) comparison in benzonitrile
[0085] To each reaction vessel of a Mettler-Toledo(R) Bohdan parallel synthesizer was added 3-bromo-5-chlorobenzonitrile (0.42 g, 1.94 mmol), 4,4-dimethyl-2-oxo- 1,4-Dihydro-2H-benzo[b][1,3]oxazine-6-boronic acid (0.45g, 2.04mmol), soda ash (0.23g, 2.17mmol), ethanol SDA 3A 190 standard strength ( 6 mL) and 0.045 g (1 mol%) of the catalyst described in Table 1.
[0086] reaction
catalyst
Product conversion %
1
5% reduced Pd / BaSO 4
85
2
5% unreduced Pd / BaSO 4
93
3
5% unreduced Pd / CaCO 3
95
[0087] The suspension was stirred vigorously and heated to 80 °C overnight. After dilution with THF (25 mL), the diluted solution was analyzed by high performance liquid chromatography (HPLC).
[0088] As shown in Table 1, the conversion of product w...
Embodiment 2
[0090] Example 2-Solvent in the preparation of 3-chloro-5-(4,4-dimethyl-2-oxo-1,4-dihydro-2H-benzo[b by Pd on carbonate or sulfate ][1,3]Oxazin-6-yl)Comparison in benzonitrile
[0091] 4,4-Dimethyl-2-oxo-1,4-dihydro-2H-benzo[b][1,3]oxazine-6-boronic acid (0.45g, 2.04mmol) in soda ash (0.23 g, 2.17mmol) and 5% Pd / BaSO 4 or 5%Pd / CaCO 3 Heating with 3-bromo-5-chlorobenzonitrile (0.42 g, 1.94 mmol) in the presence of alcohol SDA 3A 190 standard strength gave 3-chloro-5-(4,4-dimethyl-2-oxo- 1,4-Dihydro-2H-benzo[b][1,3]oxazin-6-yl)benzonitrile in good yield and purity.
[0092] When the same reaction was carried out using tetrahydrofuran (THF) instead of alcohol, 3-chloro-5-(4,4-dimethyl-2-oxo-1,4-dihydro-2H-benzo[b] [1,3]oxazin-6-yl)benzonitrile, but when 5% Pd / BaSO 4 When used as a catalyst, the above products are contaminated with raw materials. When 5%Pd / CaCO 3 When used as a catalyst, only traces of 3-chloro-5-(4,4-dimethyl-2-oxo-1,4-dihydro-2H-benzo[b][1,3] azin-6-yl)...
Embodiment 3
[0094] Example 3-solvent in the preparation of 3-chloro-5-(4,4-dimethyl-2-oxo-1,4-dihydro-2H-benzo[b][1,3] using Pd / charcoal (Oxazin-6-yl) benzonitrile comparison
[0095] 4,4-Dimethyl-2-oxo-1,4-dihydro-2H-benzo[b][1,3]oxazine-6-boronic acid (0.45g, 2.04mmol) in soda ash (0.23 g, 2.17 mmol) and 5% Pd / C catalyst in the presence of 3-bromo-5-chlorobenzonitrile (0.42 g, 1.94 mmol) in a solvent heated together to give 3-chloro-5-(4,4-di Methyl-2-oxo-1,4-dihydro-2H-benzo[b][1,3]oxazin-6-yl)benzonitrile in good yield and purity. See Table 2.
[0096] 5%Pd / C catalyst
[0097] The inventors also noticed that when the reaction was carried out in methanol, impurities were produced. Without being bound by theory, the inventors suspect that the impurity arises from the addition of methanol to the nitrile moiety in the feed and product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com