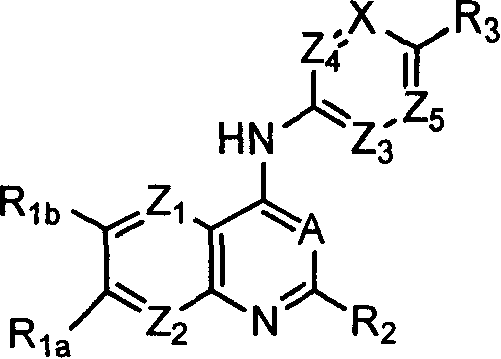
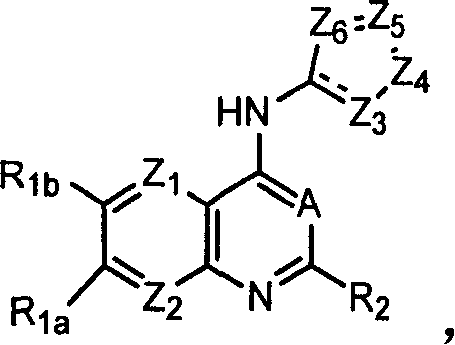
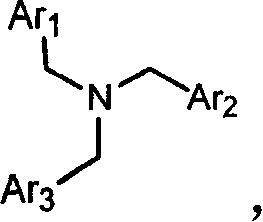
Capsaicin receptor agonists
A technology of compounds and salts, applied in the field of probes for the detection and localization of capsaicin receptors in the treatment of conditions related to capsaicin receptor activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0271] Preparation of Representative Capsaicin Receptor Agonists of Formulas Ia and Ib
[0272] A. (7-Bromo-quinazolin-4-yl)-(5-trifluoromethyl-pyridin-2-yl)-amine (Compound 1)
[0273] 1.7-Bromo-4-chloro-quinazoline
[0274]
[0275] 7-Bromo-3H-quinazolin-4-one (1.24 g, 0.0055 mol) in POCl 3 The solution was refluxed for 3.5 hours. Remove excess POCl under reduced pressure 3 , and the residue was partitioned between EtOAc and saturated NaHCO 3 between solutions. The EtOAc layer was dried and the solvent was removed under reduced pressure to afford 7-bromo-4-chloro-quinazoline as a yellow solid.
[0276] 2. (7-Bromo-quinazolin-4-yl)-(5-trifluoromethyl-pyridin-2-yl)-amine
[0277]
[0278] A mixture of 7-bromo-4-chloro-quinazoline (200 mL, 0.821 mmol) and 2-amino-5-trifluoromethyl-pyridine (239 mg, 1.48 mmol) was heated at 230°C for 2 minutes. Cool and partition the solid residue between ethyl acetate (EtOAc) and 10% NaOH. Dry the EtOAc layer (Na 2 SO ...
Embodiment 2
[0318] Preparation of Additional Representative Capsaicin Receptor Agonists of Formulas Ia and Ib
[0319] Routine modifications can be used to alter the starting materials and use additional steps to make other compounds provided in this specification, including those of Ia and Ib in the table below. In Table Ia, the compound has an EC of less than 1 micromolar when tested for capsaicin receptor agonist activity as described in Example 7. 50 .
[0320] Table Ia
[0321] Representative capsaicin receptor agonists
[0322]
[0323]
[0324]
[0325] Table Ib
[0326] Representative capsaicin receptor agonists
[0327]
[0328]
[0329]
[0330]
[0331]
[0332]
[0333]
Embodiment 3
[0335] Preparation of Representative Capsaicin Receptor Agonists of Formula II
[0336] A. 5-fluoro-1-propyl-1H-benzimidazol-2-ylmethyl-(2,4-dichloro-benzyl)-(2-ethoxy-naphthalen-1-ylmethyl )-amine (compound 55)
[0337] 1. (2,4-dichloro-benzyl)-(2-ethoxy-naphthalen-1-ylmethyl)-amine
[0338]
[0339] 2,4-Dichlorobenzylamine (500 mg, 2.81 mL) was dissolved in 2-ethoxy-1-naphthaldehyde (569 mg, 2.84 mL), acetic acid (6 drops) and tetrahydrofuran (THF) (25 ml) solution. Sodium triacetoxyborohydride (903 mL, 4.26 mmol) was added in portions, and the mixture was heated at 50°C overnight. The solvent was removed under reduced pressure, and the remaining residue was dissolved in EtOAc (25 mL) and 1N NaOH (25 mL). The organic layer was removed and the aqueous solution was extracted with an additional 25 mL of EtOAc. The two organic extracts were combined and washed with brine (50 mL). Take Na 2 SO 4The combined extracts were dried and the remaining solvent was r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com