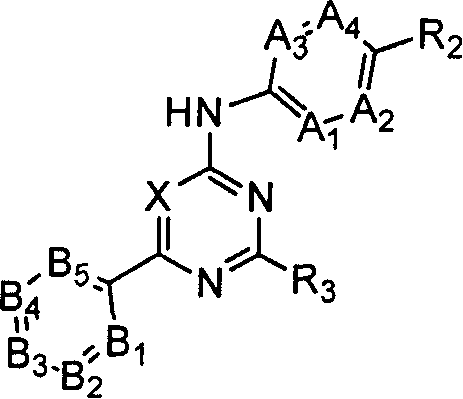
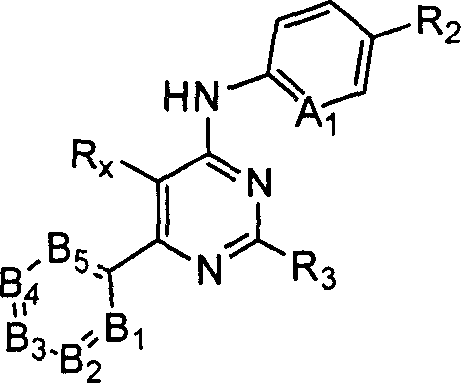
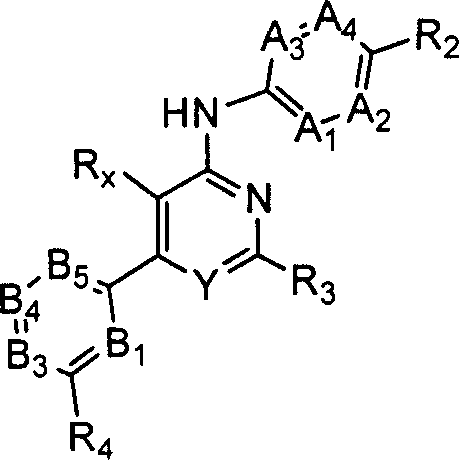
Substituted pyrimidin-4-ylamina analogues as vanilloid receptor ligands
A halogenated alkyl and alkyl technology, which can be used in the field of probes for detecting and locating capsaicin receptors, and can solve problems such as burning pain and limited therapeutic use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0326] Preparation of [4-tert-butyl-phenyl][6-(3-methoxy-phenyl)pyrimidin-4-yl]amine
[0327] This example illustrates the preparation of a representative substituted pyrimidin-4-ylamine analog: [4-tert-butyl-phenyl]-[6-(3-methoxyphenyl)pyrimidin-4-yl]amine .
[0328] 1.1-(6-chloropyrimidin-4-yl)-3-methoxybenzene
[0329]
[0330] 4,6-Dichloropyrimidine (5g, 33.5mmol), 3-methoxy-phenylboronic acid (5.17g, 34.0mmol), tris(triphenylphosphine)palladium(0) (1.4g, 1.1mmol), A mixture of 2M potassium carbonate (35 mL) in toluene (150 mL) was heated at 80° C. for 8 hours under nitrogen. The reaction mixture was then cooled and the layers were separated. The aqueous layer was extracted with ethyl acetate (3 x 100 mL), then the combined organic layers were washed with 4M NaOH (100 mL), water (100 mL) and brine (100 mL). followed by MgSO 4 After drying the organic layer, it was concentrated under reduced pressure. The concentrated residue was purified by flash chromatography (7...
Embodiment 2
[0335] Synthesis of other representative pyrimidin-4-ylamine analogs
[0336] A. [4-tert-butyl-phenyl]-[6-(3-methoxyphenyl)-5-methyl-2-morpholin-4-ylpyrimidin-4-yl]amine
[0337] 1.5-Methyl-2-morpholin-4-ylpyrimidine-4,6-diol
[0338]
[0339] A mixture of sodium methoxide (15mL, 45mmol), morpholinoformamidine hydrobromide (6.3g, 30mmol) and diethyl methylmalonate (5.22g, 30mmol) dissolved in methanol was heated at 50°C Heat for 2 hours. After cooling, it was concentrated under reduced pressure. The concentrated white gum was dissolved in water and acidified with concentrated sulfuric acid. The resulting white solid was collected by filtration, washed with water and air dried to give the title compound.
[0340] 2.4-(4,6-dichloro-5-methylpyrimidin-2-yl)morpholine
[0341]
[0342] 5-Methyl-2-morpholin-4-ylpyrimidine-4,6-diol (3.57g, 17mmol), N,N-diethylaniline (4.37g, 35mmol) and phosphorus oxychloride (phosphorus oxychloride) (25 mL) was heated at 90°C for 2 hours...
Embodiment 3
[0360] Other representative substituted pyrimidin-4-ylamine analogs
[0361] Various changes may be made to the starting materials and other procedures may be used to produce other compounds provided herein by using routine modifications. The compounds listed in Table I were prepared using the method described. In marked "IC 50 "*" in the column of the field indicates the IC determined as described in Example 6 50 at a concentration of 1 micromolar or less (i.e., cells exposed to IC 50 The concentration of capsaicin required to reduce the fluorescent response produced by capsaicin by 50% is 1 micromolar or less). The mass spectrometry data in the columns marked with the "MS" field are electrospray mass spectrometry (Electospray MS) data, in positive ion mode with a conical voltage of 15 or 30 volts (V), by using a Waters 600 motor, Waters Micromass Time-of-Flight LCT (Micromass Time-of-Flight LCT) of 996-type photodiode array detector, Gilson215 automatic sampler and Gilso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com