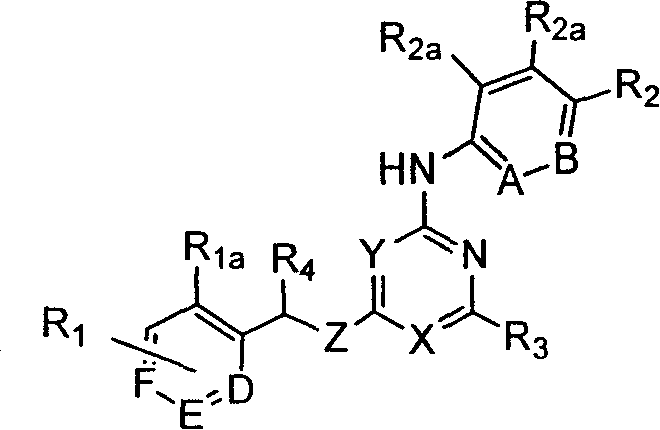
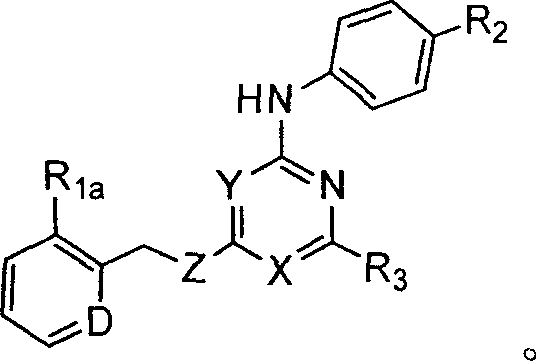
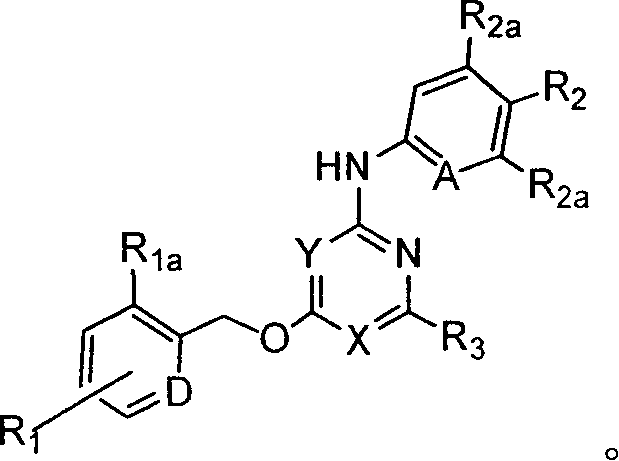
Substituted pyridin-2-ylamine analogues
A technology of substituents and haloalkyl groups is applied in the field of probes for detecting and locating capsaicin receptors, and can solve problems such as limited therapeutic use and burning pain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0283] Preparation of representative substituted pyridin-2-ylamine analogs
[0284] This example illustrates representative substituted pyridin-2-ylamine analogs.
[0285] A. [4,6-bis(2-trifluoromethyl-benzyloxy)-[1,3,5]triazin-2-yl]-(4-trifluoromethyl-phenyl)amine
[0286] 1. (4,6-dichloro-[1,3,5]triazin-2-yl)-(4-trifluoromethyl-phenyl)amine
[0287]
[0288] Add diisopropylethylamine (1.39 g, 0.0108 mol). After adding 4-trifluoromethyl-phenylamine (1.74 g, 0.0108 mol) dropwise to the mixture, the reaction was stirred at 0° C. for 2 hours, and then at room temperature for 16 hours. After diluting the reaction mixture with ethyl acetate, water (2x), saturated NaHCO 3 (1x) and brine (1x) washes. Organic layer with Na 2 SO 4 After drying, concentration was performed under reduced pressure. The concentrated residue was purified by preparative plate chromatography (eluent: 20% ethyl acetate / hexane) to give the title compound.
[0289] 2. [4,6-bis(2-trifluoromethyl-benz...
Embodiment 2
[0320] N 4 Preparation of -(4-tert-butyl-phenyl)-6-(2-trifluoromethyl-benzyloxy)-pyrimidine-2,4-diamine
[0321] 1.N 4 -(4-tert-butyl-phenyl)-6-chloro-pyridine-2,4-diamine
[0322]
[0323] After adding 4-tert-butyl-phenylamine (1.82 g, 0.0122 mol) to a solution of 4,6-dichloro-pyrimidin-2 ylamine (2.0 g, 0.0122 mol) in acetonitrile, the mixture was stirred at 70° C. 16 hours. Then cooled to room temperature, concentrated, in saturated NaHCO 3 Partition between aqueous solution and ethyl acetate. The organic layer was washed with brine, washed with Na 2 SO 4 Dry, then concentrate under reduced pressure. The concentrated residue was purified by flash chromatography (mobile phase 25% ethyl acetate / hexanes) to afford the title compound.
[0324] 2.N 4 -(4-tert-butyl-phenyl)-6-(2-trifluoromethyl-benzyloxy)-pyrimidine-2,4-diamine
[0325]
[0326] NaH (51 mg, 1.28 mmol, 60% suspension in mineral oil) was added to a solution of (2-trifluoromethyl-phenyl)methanol (300...
Embodiment 3
[0328] Representative substituted pyridin-2-ylamine analogs
[0329] Various changes may be made to the starting materials and other procedures may be used to produce other compounds provided herein by using routine modifications. The compounds listed in Table I were prepared using the method described. In marked "IC 50 "*" in the column of the field indicates the IC determined as described in Example 6 50 at a concentration of 1 micromolar or less (i.e., cells exposed to IC 50 The concentration of capsaicin required to reduce the fluorescent response produced by capsaicin by 50% is 1 micromolar or less). The mass spectrometry data in the columns marked with the "MS" field are electrospray mass spectrometry (Electospray MS) data, in positive ion mode with a conical voltage of 15 or 30 volts (V), by using a Waters 600 motor, Waters Micromass Time-of-Flight LCT (Micromass Time-of-Flight LCT) of 996-type photodiode array detector, Gilson215 automatic sampler and Gilson 841 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com