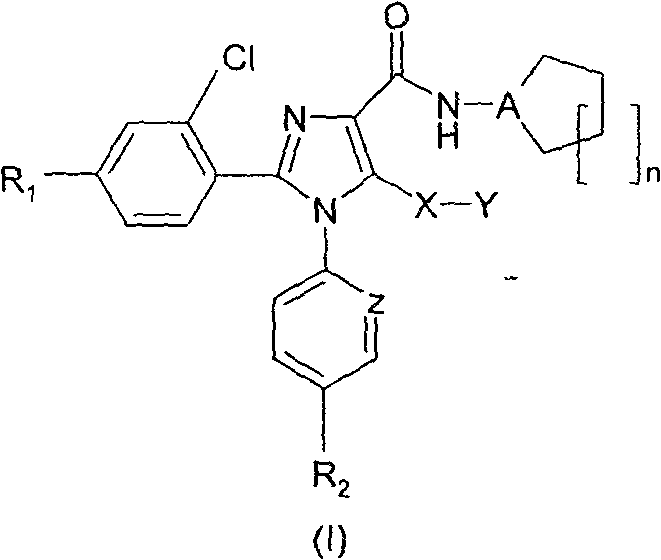
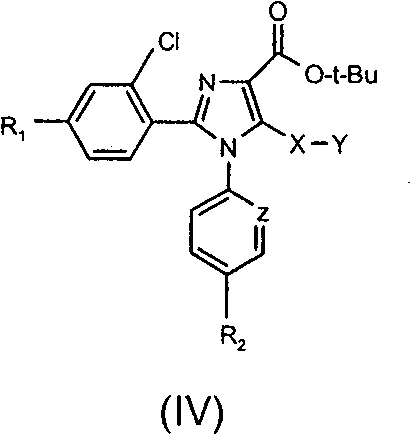
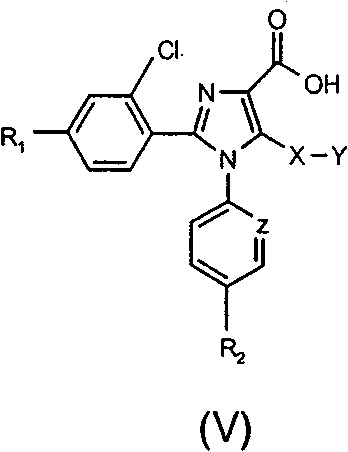
Tetrasubstituted imidazole derivatives as cannabinoid cb1 receptor modulators with a high CB1/CB2 receptor subtype selectivity
A technology for stereoisomers and drugs, applied in the field of imidazole derivatives, can solve problems such as no disclosure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Materials and methods
[0052] On Bruker Avance DRX600 instrument (600MHz), Varian UN400 instrument (400MHz) or Varian VXR200 instrument (200MHz), use DMSO-d 6 or CDCl 3 As solvent and tetramethylsilane as internal standard, record 1 H and 13 C NMR spectrum. Chemical shifts are given in ppm (delta scale) downfield from tetramethylsilane. Coupling constants (J) are expressed in Hz. Flash chromatography was performed using silica gel 60 (0.040-0.063 mm, Merck). Column chromatography was performed using silica gel 60 (0.063-0.200 mm, Merck). Melting points were recorded using a Büchi B-545 melting point apparatus. Mass spectra were recorded on a Micromass QTOF-2 instrument with MassLynx application software for data acquisition and reconstruction. Perform quasi-molecular ions [M+H] + exact mass determination.
Embodiment 2
[0053] Embodiment 2: the synthesis of concrete compound
[0054] Compound 1-3
[0055] 1-(4-Chlorophenyl)-2-(2,4-dichlorophenyl)-1H-imidazole-4-carboxylic acid
[0056] To magnetically stirred ethyl 1-(4-chlorophenyl)-2-(2,4-dichlorophenyl)-1H-imidazole-4-carboxylate (18.44 g, 0.0466 mol) in THF (240 ml) LiOH (2.24g, 0.0932mol) and H were added to the solution 2 O (240ml). The resulting mixture was stirred at 50 °C for 16 hours to give a clear solution. After cooling to room temperature, HCl (1N solution, 95ml) and H 2 O (240ml) gave a precipitate which was collected by filtration, washed with water and dried in vacuo to give 1-(4-chlorophenyl)-2-(2,4-dichlorophenyl)-1H-imidazole- 4-Formic acid (16.83 g, 98% yield), mp 138-142°C (decomposition); 1 H-NMR (600MHz, DMSO-d 6 )δ7.08(br d, J=8Hz, 2H), 7.31-7.37(m, 4H), 7.45(d, J=8Hz, 1H), 7.96(s, 1H); 13 C-NMR (150MHz, DMSO-d 6 )δ 126.87, 127.85, 127.91, 128.47, 129.36, 129.66, 133.56, 133.99, 134.44, 134.49, 135.54, 135.99...
Embodiment 4
[0093] Example 4: Formulations for Animal Studies
[0094] Oral (p.o.) administration: To the desired amount (0.5-5 mg) of solid Compound 1 in a glass tube was added a certain amount of glass beads and the solid was triturated by vortexing for 2 minutes. After adding 1 ml of a solution of 1% methylcellulose in water and 2% (v / v) poloxamer 188 (Lutrol F68), the compound was suspended by vortexing for 10 minutes. The pH was adjusted to 7 with a few drops of aqueous NaOH (0.1 N). The remaining particles in suspension were further suspended by using an ultrasonic bath.
[0095] Intraperitoneal (i.p.) administration: To the desired amount (0.5-15 mg) of solid Compound 1 in a glass tube was added a certain amount of glass beads and the solid was triturated by vortexing for 2 minutes. After adding 1 ml of a solution of 1% methylcellulose and 5% mannitol in water, the compound was suspended by vortexing for 10 minutes. Finally the pH was adjusted to 7.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com