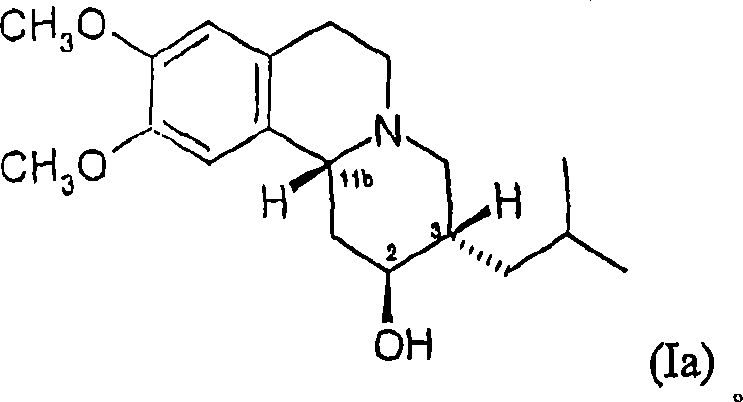
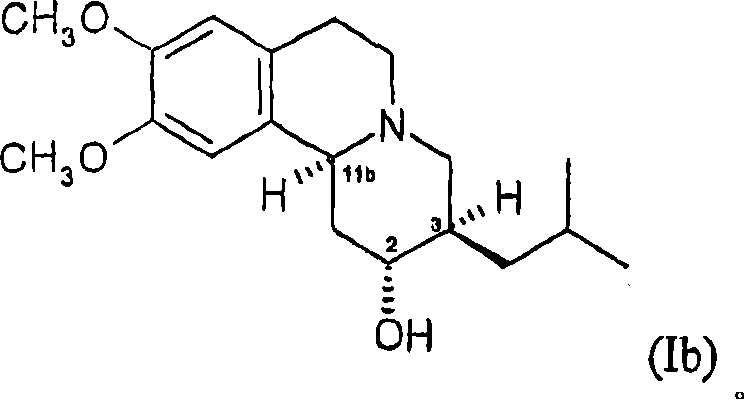
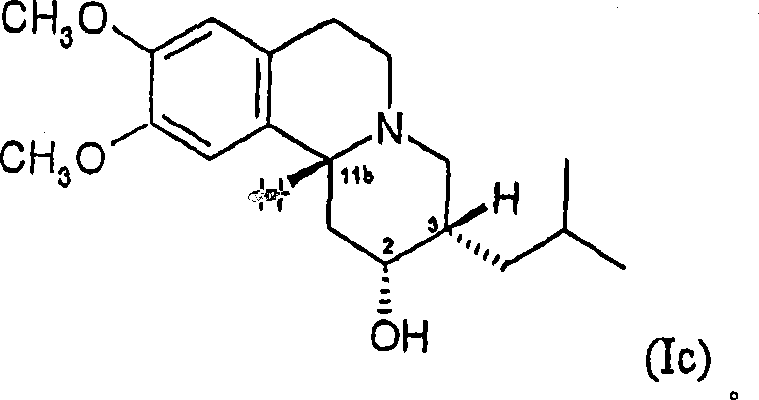
Dihydrotetrabenazines and pharmaceutical compositions containing them.
A technology of dihydrotetrabenazine and dihydrobutadiene, applied in the field of dihydrotetrabenazine isomers, can solve problems such as unstable tetrabenazine and biological activity of unpublished compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Preparation of 2S, 3S, 11bR and 2R, 3R, 11bS Isomers of Dihydrotetrabenazine 1A. Reduced RR / SS Tetrabenazine
[0133]
[0134] 1M L-Selectride in tetrahydrofuran (135ml, 135mmol, 2.87eq) was dissolved at 0°C over 30 minutes To a stirred solution of tetrabenazine RR / SS racemate (15 g, 47 mmol) in ethanol (75 ml) and tetrahydrofuran (75 ml) was added slowly. The mixture was stirred at 0 °C for 30 min after complete addition and then allowed to warm to room temperature.
[0135] The mixture was poured onto crushed ice (300g) and water (100ml) was added. The solution was extracted with diethyl ether (2 x 200ml) and the combined ethereal extracts were washed with water (100ml) and partially dried over anhydrous potassium carbonate. Drying was completed with anhydrous magnesium sulfate, and after filtration, the solvent was removed under reduced pressure (protected from light, bath temperature <20°C) to obtain a pale yellow solid.
[0136] The solid was slurried w...
Embodiment 2
[0164] Preparation of 2R, 3S, 11bR and 2S, 3R, 11bS Isomers of Dihydrotetrabenazine 2A. Preparation of 2,3-dihydrotetrabenazine
[0165] According to the method of embodiment 1A, use L-Selectride Reduction of a THF solution containing a racemic mixture of RR and SS tetrabenazine enantiomers (15 g, 47 mmol) afforded a mixture of 2S, 3R, 11bR and 2R, 3S, 11bS enantiomers of dihydrotetrabenazine (12 g , 80%), as a white powdery solid. Then according to the method of embodiment 1B, with PCl 5 Dehydration of the partially purified dihydrotetrabenazine afforded a semi-purified mixture of the 11bR and 11bS isomers of 2,3-dihydrotetrabenazine (the 11bR enantiomers are not shown below) (12.92 g, 68% ), as a yellow solid.
[0166]
[0167] 2B. Epoxidation of the Crude Olefin from Example 2A
[0168]
[0169] A solution of 70% perchloric acid (3.70ml, 43mmol) in methanol (215ml) was added to a stirred solution of the crude olefin from Example 2A (12.92g, 42.9mmol) in me...
Embodiment 3
[0203] Alternative Methods for the Preparation of Isomer B and the Preparation of the Mesylate Salt
[0204] 3A. Reduced RR / SS Tetrabenazine
[0205]
[0206] 1M Selectride in tetrahydrofuran (52ml, 52mmol, 1.1 equivalents) was added in 30 minutes To a cooled (ice bath), stirred solution of tetrabenazine racemate (15 g, 47 mmol) in tetrahydrofuran (56 ml) was added slowly. After the addition was complete, the mixture was allowed to warm to room temperature and stirred for an additional 6 hours. TLC analysis (silica, ethyl acetate) showed very little starting material remaining.
[0207] The mixture was poured into a stirred mixture of crushed ice (112g), water (56ml) and glacial acetic acid (12.2g). The resulting yellow solution was washed with diethyl ether (2 x 50ml) and basified by the slow addition of solid sodium carbonate (ca. 13g). Pet-ether (30-40°C) (56ml) was added to the mixture with stirring and the crude product β-DHTBZ was collected by filtration as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com