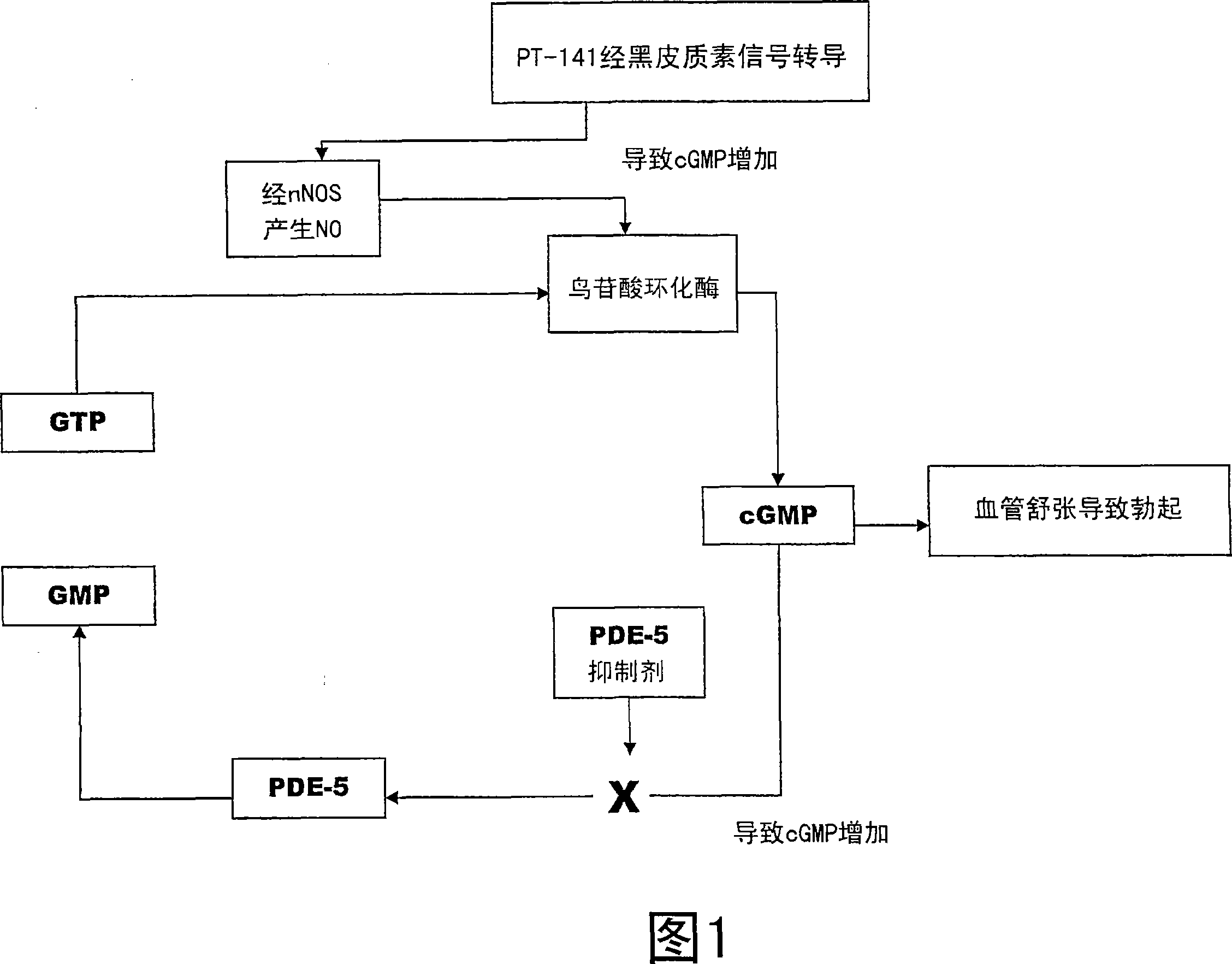
Multiple agent therapy for sexual dysfunction
A technology for erectile dysfunction and sexual function, applied in the field of multi-drug therapy for sexual dysfunction, and can solve problems such as dose-dependent side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
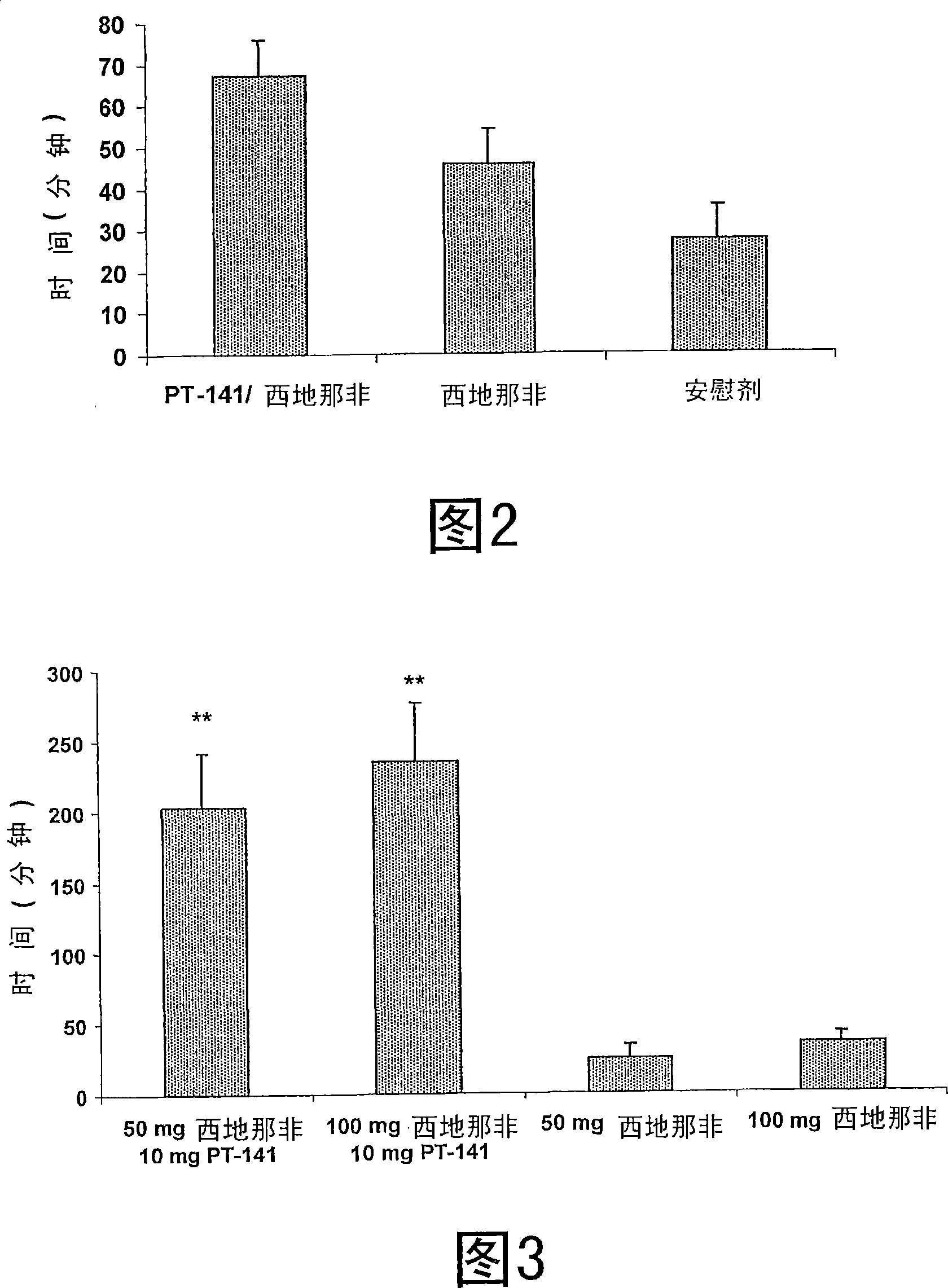
[0132] In human clinical studies, a group of nineteen male patients with erectile dysfunction was evaluated in a placebo-controlled, randomized double-blind, three-step crossover trial. Each clinical study participant received in a random manner: (1) 25 mg sildenafil, 7.5 mg PT-141 administered IN after 5 minutes (test 1), (2) 25 mg sildenafil, no PT-141 ( Test two), and (3) give placebo only (test three). Select individuals who have been diagnosed with erectile dysfunction and respond to sildenafil or vardenafil, typically 50 to 100 mg of sildenafil. Placebo treatment consisted of a placebo tablet and a placebo spray; in Test 2 and Test 3, placebo was given appropriately so that the study participants were unaware of receiving the oral form of sildenafil or the placebo tablet Keep it ignorant.
[0133] During the study, connect the RigiScan described below 30 minutes (T-30) before dosing A device in which the patient is administered at T0, 50 to 80 minutes (T50 to T80) after the...
Embodiment 2
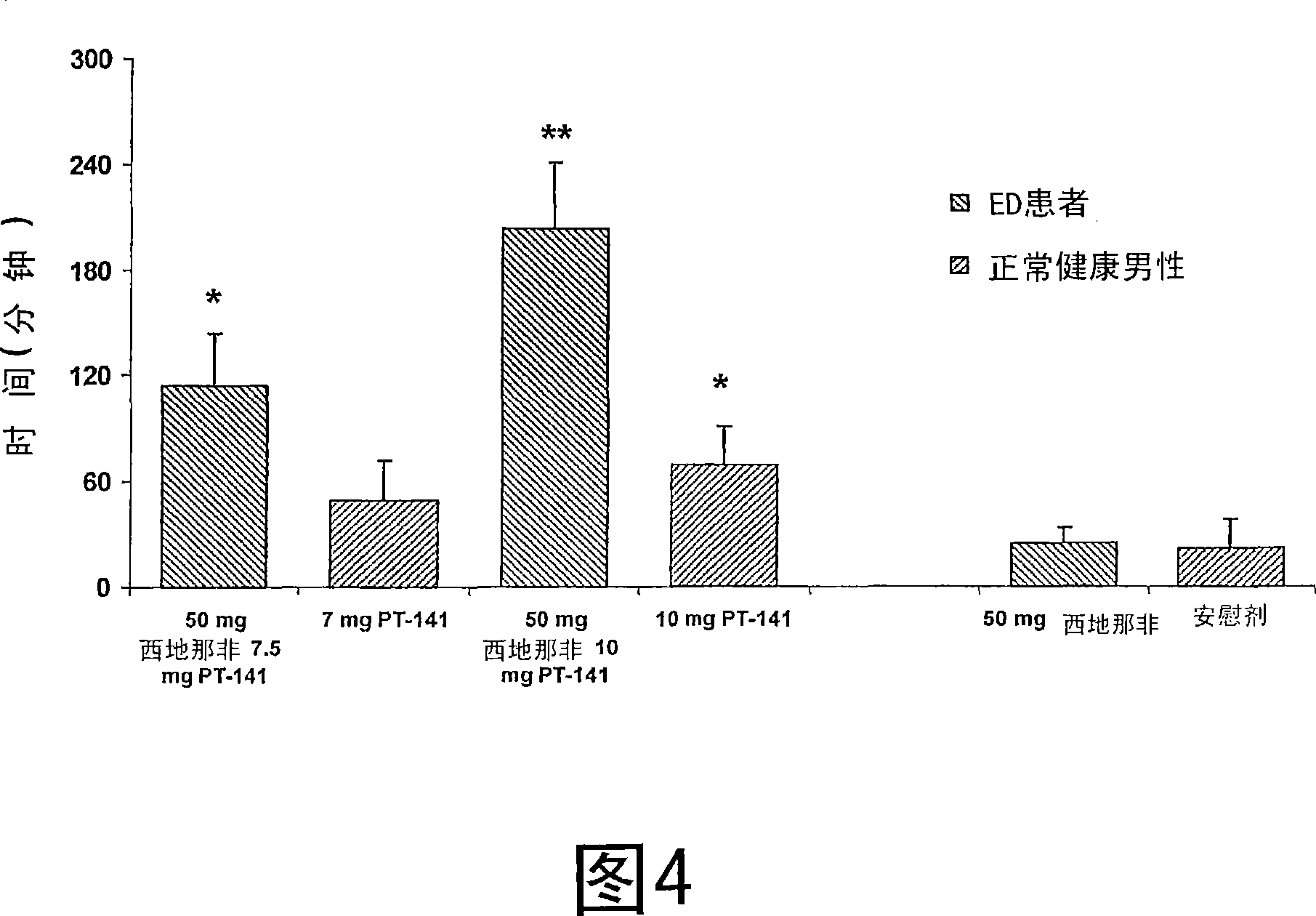
[0144] In the second human clinical study, patients with erectile dysfunction were evaluated in a placebo-controlled, randomized double-blind, escalating dose drug interaction study involving the combined administration of IN PT-141 and sildenafil. A group of thirty-two male patients. Individuals in each dose group received a single dose of sildenafil in a 3:1 ratio in combination with a single dose of IN PT-141 or placebo spray; in each dose group of 8 individuals, 6 One subject received sildenafil and PT-141, and two subjects received sildenafil and placebo spray. Sildenafil doses are 50mg and 100mg. The doses of PT-141 are 7.5mg and 10mg. Only if all individuals in the dose group have received the study drug and have tolerated the current dose level satisfactorily, will the dose be increased to the next higher dose group. Research measurements include vital signs, ECG evaluation, spontaneous adverse event (AE) report and use of RigiScan Monitor for a total of six and a half h...
Embodiment 3
[0170] The study was conducted on normal volunteers, and other aspects were the same as the protocol of Example 2, and the data obtained was compared with the data obtained in Example 2. Therefore, the only difference is that normal volunteers are study individuals, and they respond more to erectile drugs than people diagnosed with erectile dysfunction. Give normal volunteers 7mg or 10mg IN PT-141, and test according to Example 2, using RigiScan The monitoring is for a total of six and a half hours. For the measurement of the duration of the bottom hardness ≥ 60%, compared with the results obtained with any of 50 mg sildenafil, 100 mg sildenafil, 7 mg IN PT-141 or 10 mg IN PT-141, 50 mg of sildenafil The results obtained with denafil and 10 mg IN PT-141 or 100 mg sildenafil and 10 mg IN PT-141 (both patients with erectile dysfunction) were statistically significantly higher (p<0.05). The results are shown in Figure 4. Similarly, for the measurement of the duration of the bottom ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com