Immunoglobulins
An antibody, therapeutic antibody technology, applied in the field of immunoglobulin, which can solve the problems of low potency, slow onset, and poor tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
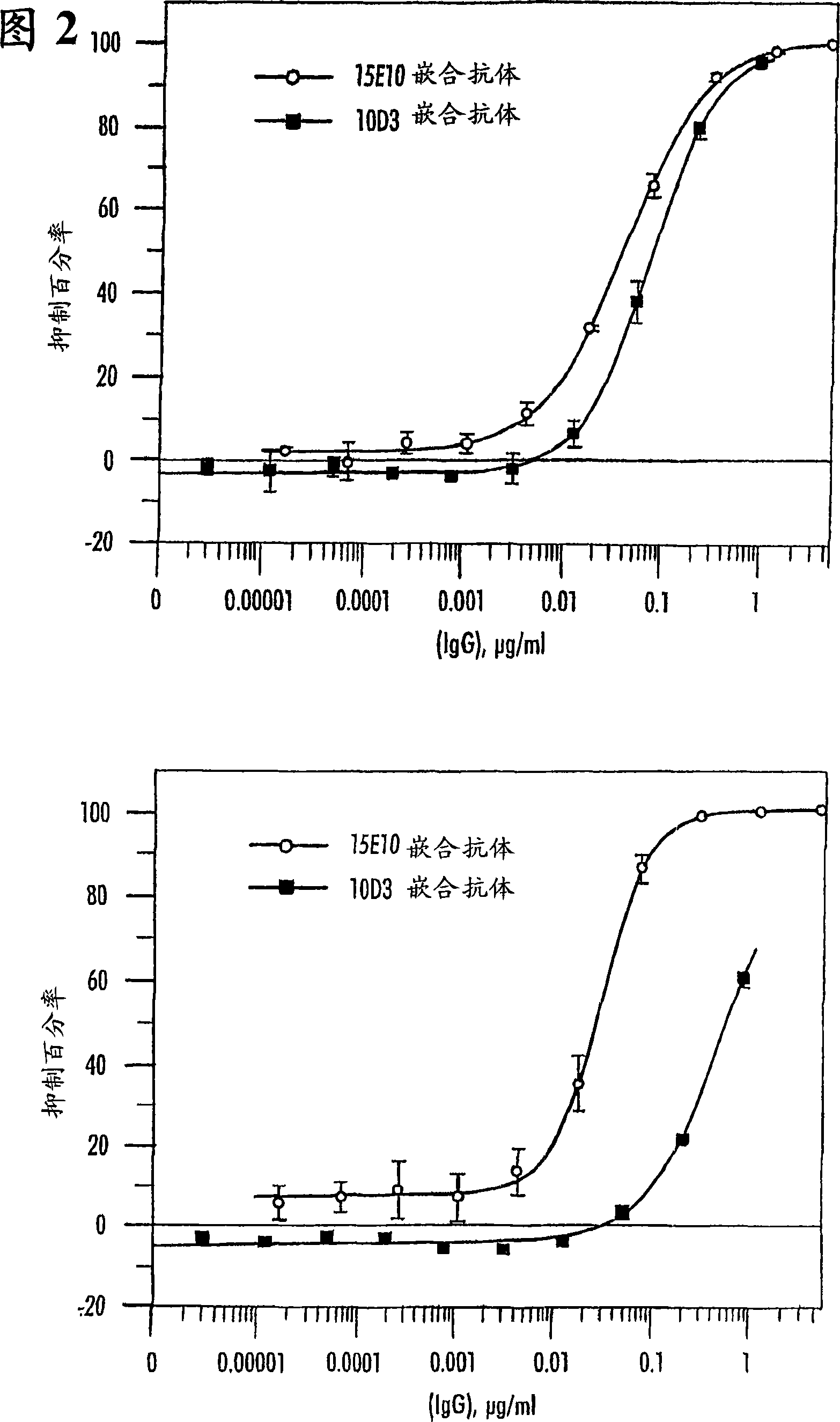
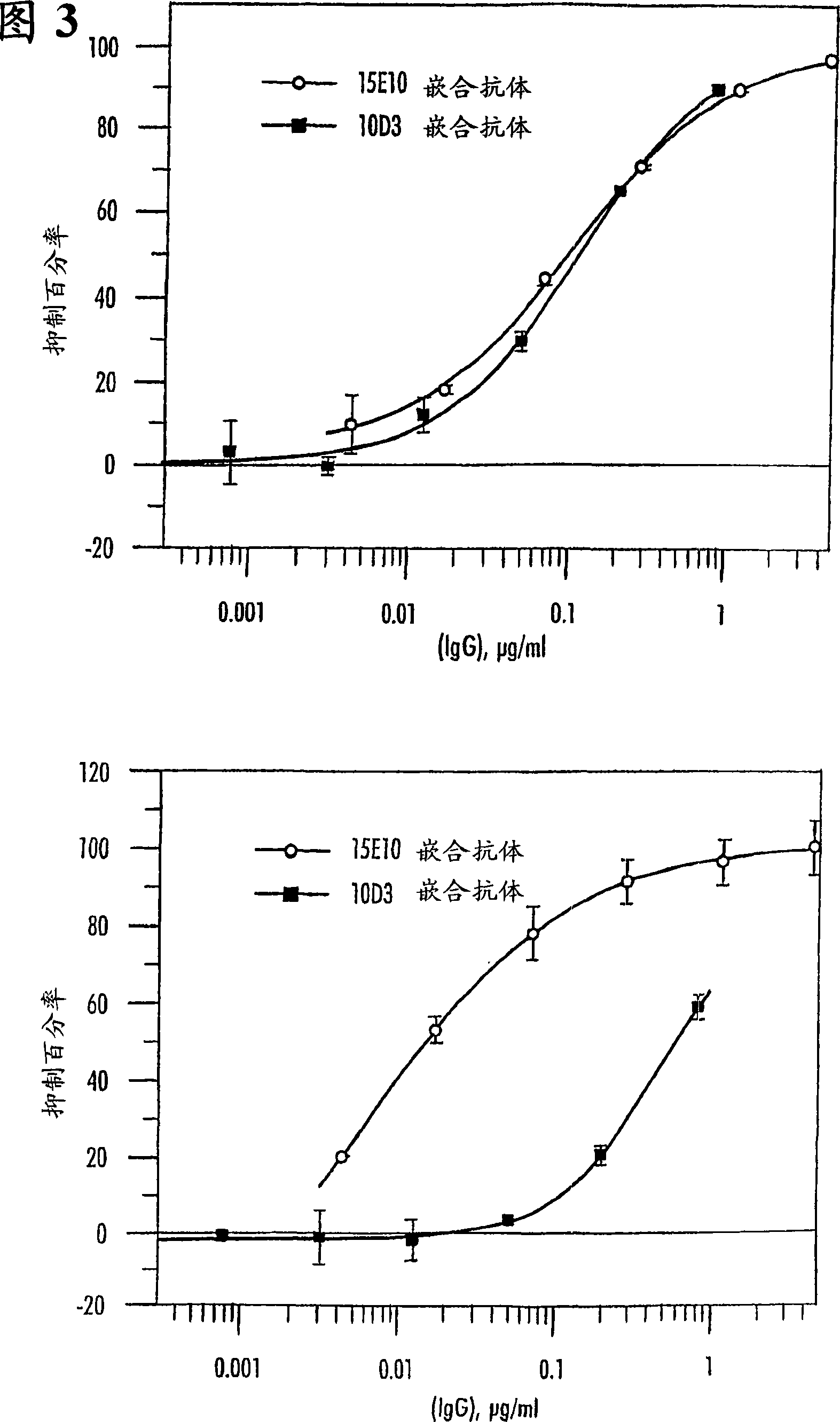
[0332] Examples 1-6 relate to the production and engineering of antibody 15E10. Example 7 relates to the production and engineering of antibody 10D3.
[0333] 1. Production of monoclonal antibodies
[0334] Monoclonal antibodies are generally produced from hybridomas as described in E Harlow and D Lane, Antibodies a Laboratory Manual, Cold Spring Harbor Laboratory, 1988 . Fusion of mouse myeloma cells with B lymphocytes from mice immunized with the target antigen. The myeloma fusion partner immortalizes the hybridoma cells while providing antibody production capacity by the B lymphocytes.
[0335] Four SJL mice were immunized by intraperitoneal injection of a suspension of glycosylated human OSM (hOSM) produced in CHO cells in RIBI adjuvant (Sigma). After 2 weeks, mice were boosted with hOSM alone, followed, after a further 2 weeks, with hOSM neutralized with an anti-site III monoclonal antibody (OM4 / 11.17; OSM:Mab 1:1.5 wt:wt). Mice were boosted with hOSM to direct the ...
Embodiment 7
[0693] Example 7 - Antibody 10D3
[0694] 7.1. Production of monoclonal antibodies
[0695] Hybridoma 10D3 was generated as detailed in Example 1 above.
[0696] 7.2. Cloning of the 10D3 variable region
[0697] Total RNA was extracted from clone 10D3 hybridoma cells, and cDNAs for the variable domains of the heavy and light chains were generated by reverse transcription using murine leader-specific primers and antibody constant regions of the predetermined isotype (IgG1 / κ). The cDNAs of the heavy and light chain variable domains were then cloned into vector pCR2.1 for sequencing.
[0698] 7.2.1 RNA extraction
[0699] 10 of 10D3 were cloned from hybridomas using Promega's SV Total RNA Isolation System following the manufacturer's instructions. 6 Total RNA was extracted from the cell pellet.
[0700] 7.2.2 Reverse transcription
[0701] RNA was reverse transcribed using primers specific for the murine leader sequence and murine IgG gamma 2a / kappa constant regio...
Embodiment 8
[0885] Example 8. gp130 inhibition ELISA
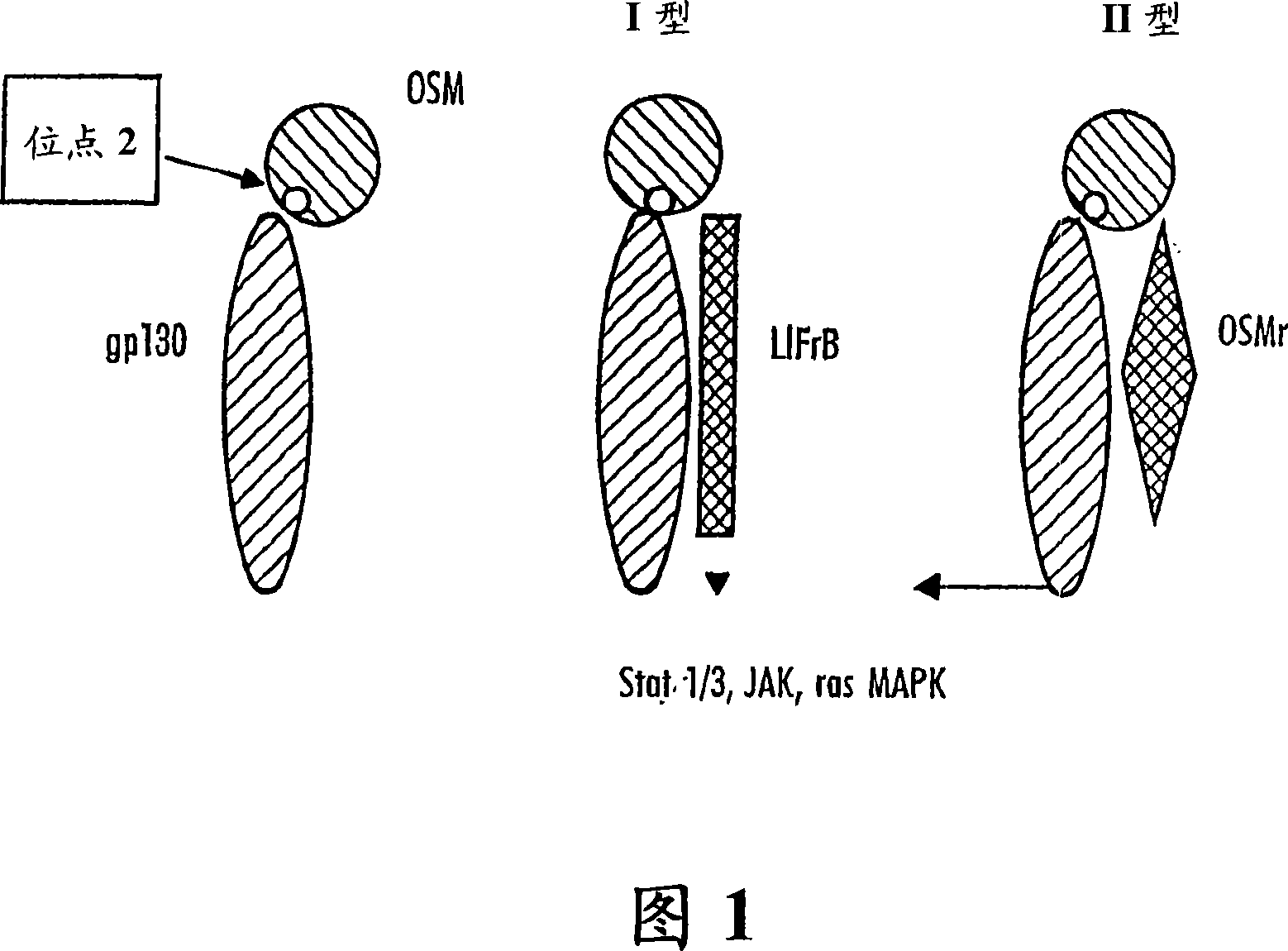
[0886] OSM sequentially binds gp130 and either the OSM receptor or the LIF receptor. The experiments described here allow the measurement of OSM (eg hOSM) bound to gp130 on ELISA plates. In addition, this experiment allows measuring the inhibition of OSM binding to the gp130 receptor by antibodies directed against OSM site II.
[0887] 8.1 Materials
[0888] 1. Nunc Immunoplate 1 F96 Maxisorp (Life Technologies, 4-39454A)
[0889] 2. Human gp130-Fc 100μg / ml (R&D Systems, 671-GP-100)
[0890] 3. PBS
[0891] 4.BSA (Sigma A7030)
[0892] 5. Human recombinant OSM 10 μg / ml (R&D Systems, non-glycosylated)
[0893] 6. Biotinylated anti-human OSM 50 μg / ml (R&D Systems, BAF295)
[0894] 7. Streptavidin HRP (Amersham RPN4401)
[0895] 8. 3,3′,5,5′-Tetramethylbenzidine (TMB) (Sigma)
[0896] 9. Sulfuric acid
[0897] 10. Tween 20 (Sigma P7949)
[0898] 8.2 Preparation of reagents
[0899] 1. Plate preparation: Human gp130-Fc w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com