Methods and compositions for reducing redundant molecular barcodes created in primer extension reactions
a primer extension and barcode technology, applied in the field of nucleotide sequence amplification, can solve the problems of reducing affecting the amplification efficiency of primer extension reactions, so as to reduce the number of molecular barcodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Dual Molecular Barcodes
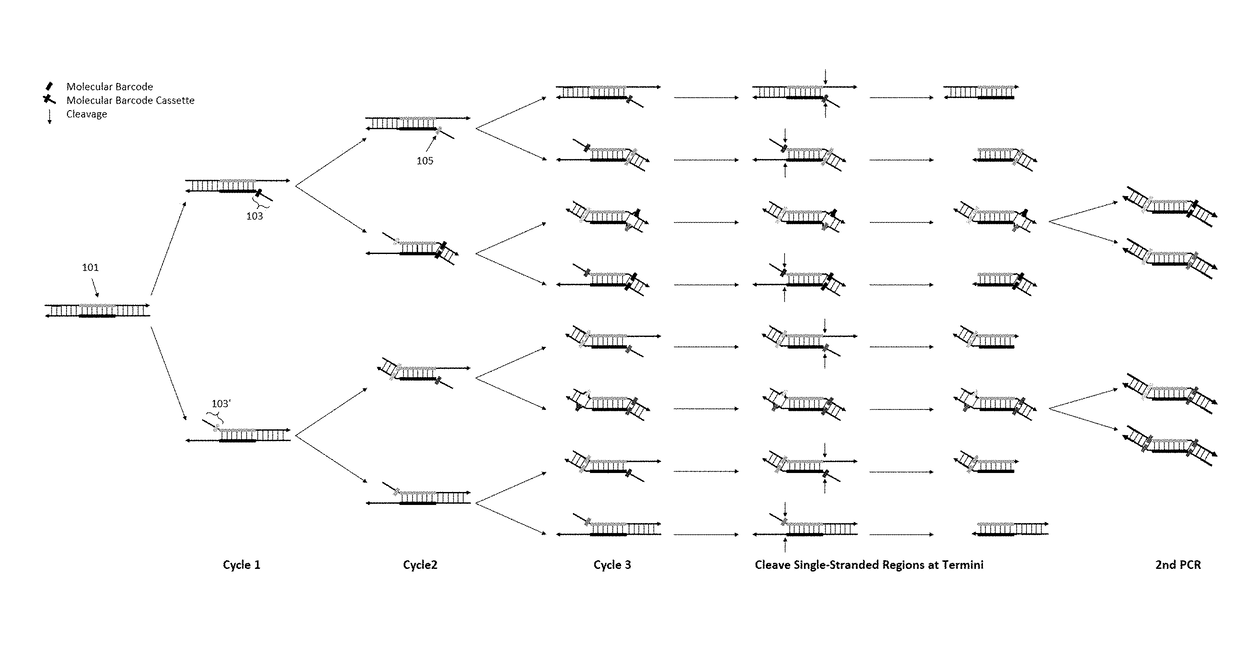
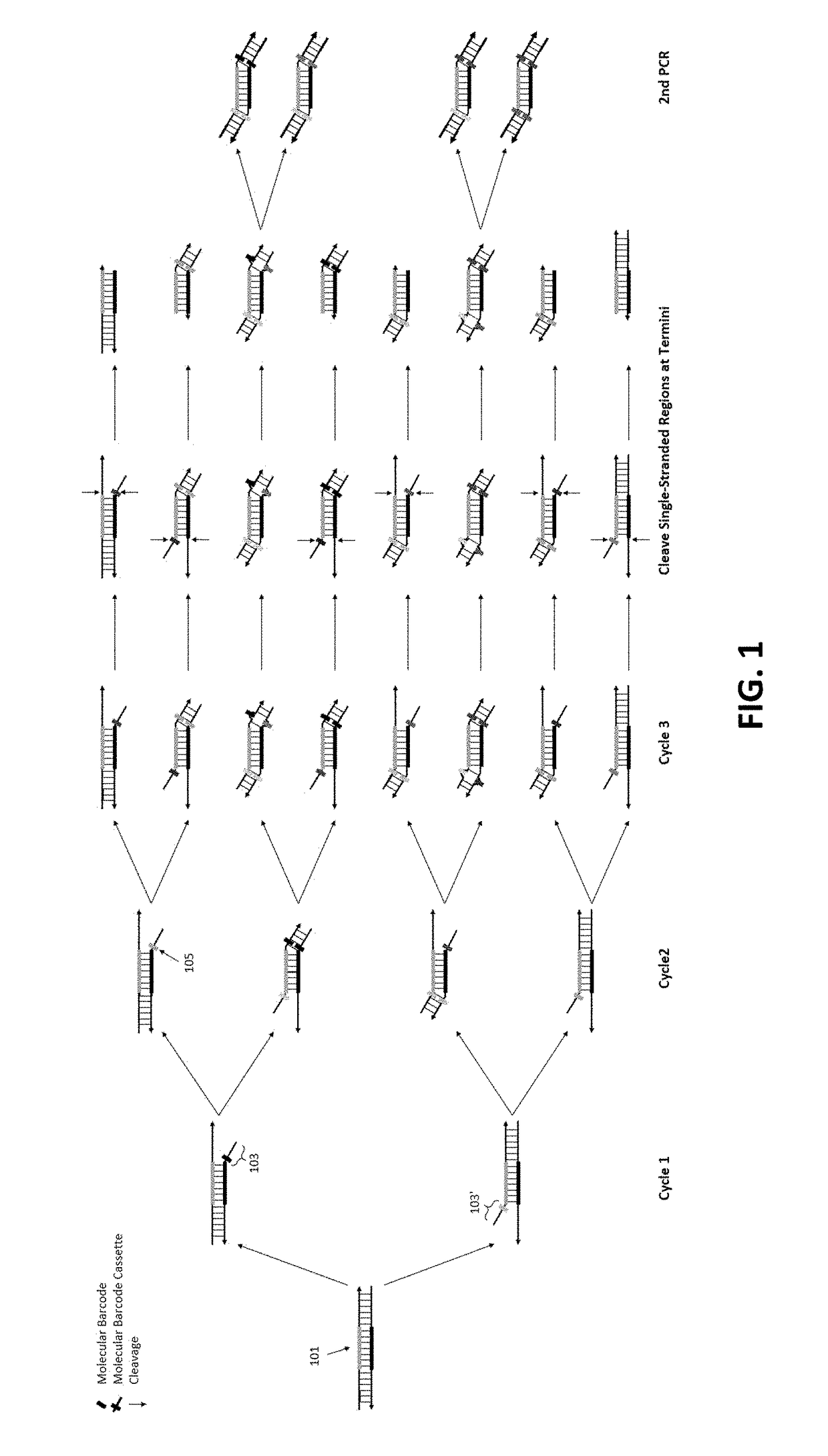
[0101]To demonstrate the feasibility of our method of removing redundant molecular barcodes from a primer extension reaction involving molecular barcodes on BOTH primers (depicted in FIG. 1), and the consequent removing of random errors and variant calling from both strands of target DNA (FIG. 3), the effect of single-stranded DNA specific exonuclease on the yield of DNA libraries was tested, and the subsequent variant calling after sequencing the libraries.
[0102]To enable a library to be sequenced in Illumina sequencing machines, the library structure was designed as shown in FIG. 6A. DNA targets were amplified with a plurality of pairs of primers (also referred as primer panel or panel) in the multiplex PCR reaction for three cycles. After removing redundant barcodes, the products of multiplex PCR were further amplified in the second round of PCR with a pair of primers to produce the library. A primer panel containing 40 pairs of primers (40 plex) was used. ...
example 2
Single Molecular Barcode
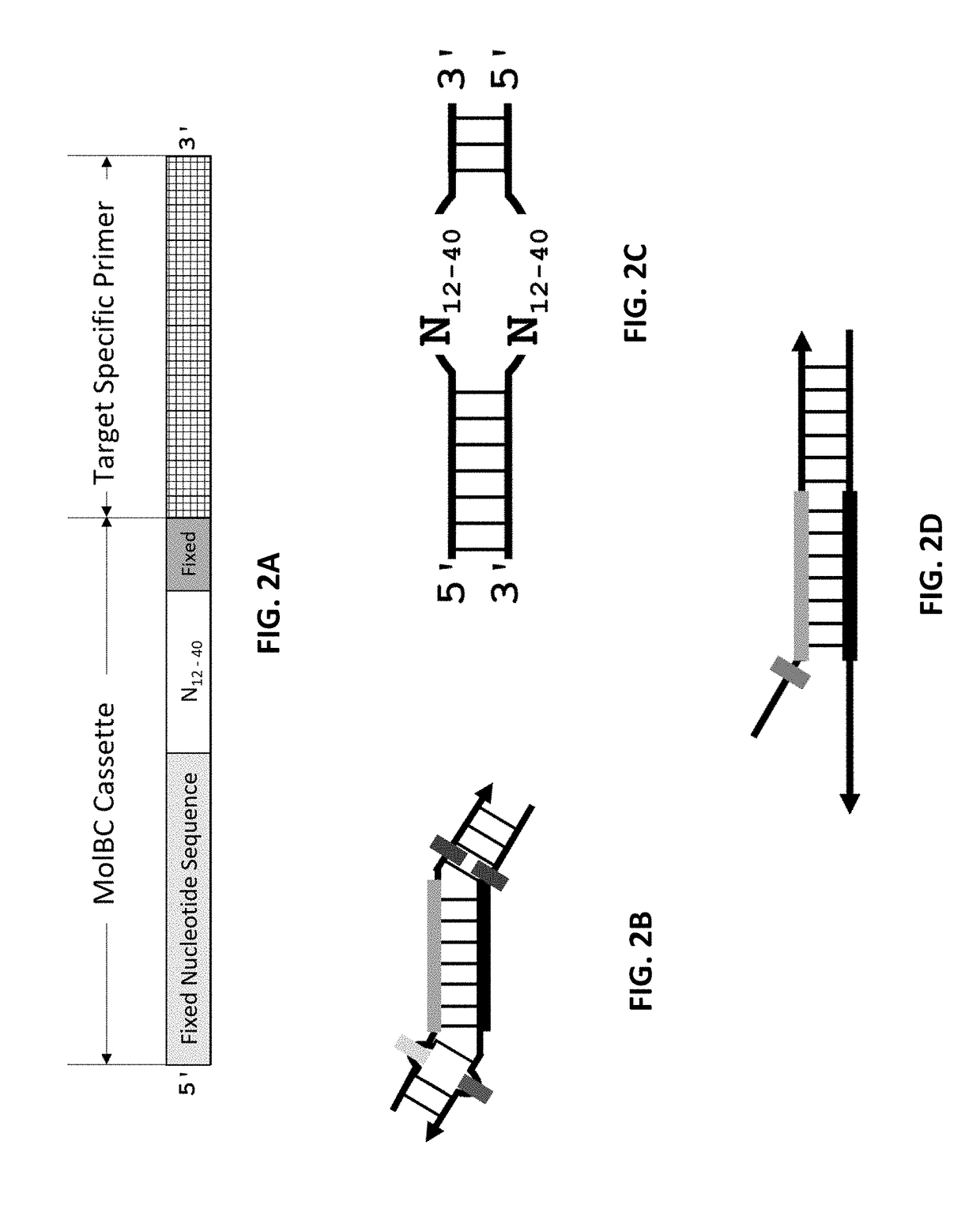
[0110]In this example, the feasibility of the method to remove redundant molecular barcodes from a primer extension reaction involving molecular barcode on ONE primer was demonstrated, as depicted in FIGS. 4 and 5. A primer panel containing 205 pairs of primers were used to amplify corresponding DNA regions in 0.2% NA12878 / NA18507. Each primer of these 205 pairs of primers contained a 3′ end target-specific nucleotide sequence and a 5′ fixed nucleotide sequences. The 5′ fixed nucleotide sequences were complimentary to the pair of primers used in the secondary PCR. Each forward primer additionally contained a 10-nucleotide random sequence, serving as molecular barcode, between the 3′ end target-specific sequence and the 5′ end fixed nucleotide sequence. 100 ng of 0.2% NA12878 / NA18507 and 25 nM each of the 205 pairs of primers were used in a multiplex PCR reaction. The reagents and the method of multiplex PCR were provided by Paragon Genomics Inc. (CleanPlex™ T...
example 3
y of Amplification
[0114]Uniformity is a measure of how well every amplicon in a library is equally amplified in a multiplex PCR reaction. In order words, it measures the difference of copy numbers of amplicons between the under-amplified amplicons and the over-amplified amplicons. We found that the 40-plex panel and 205-plex panel used in Example 1 and 2 did not properly measure the uniformity. These two panels easily generated highly uniform libraries (FIG. 18, table 1). The number of amplicons defined by these panel were not sufficiently large to accommodate various kinds of easy and difficult-to-amplify amplicons. We then used a panel of 629 pairs of primer and made libraries with the reagent used above. We sequenced these libraries at mean reads around 700. The uniformity measured at 0.2× mean reads was larger than 99%, while uniformity at 0.5× mean reads was higher than 87%. Furthermore, amplicons with GC content from over 20% to 80% were equally amplified. Three amplicons cove...
PUM
| Property | Measurement | Unit |
|---|---|---|
| work time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com