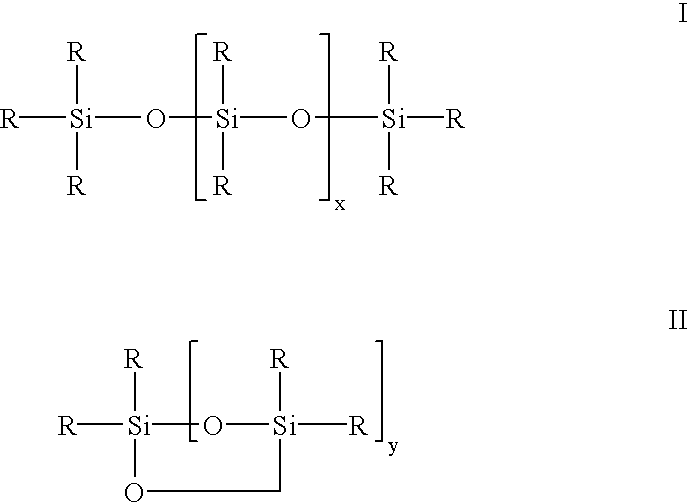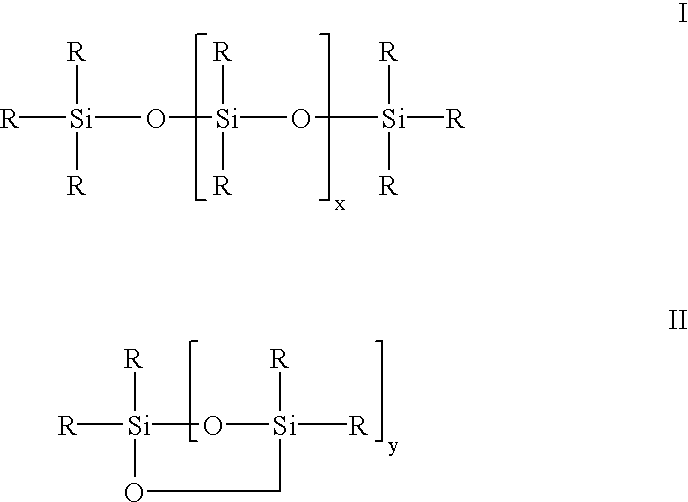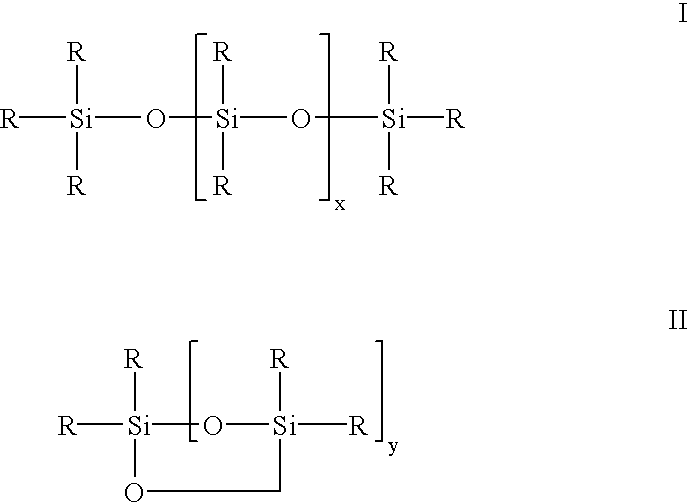Siloxane oligomers, a process for their production and their use
a technology of siloxane oligomers and production processes, applied in the field of siloxane oligomers, can solve the problems of siloxane oligomers, release of considerable amounts of alcohol during the mixing process,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Reaction of a Chloropropyltrichlorosilane-Propyltrichlorosilane Mixture with Water and Ethanol
[0049] 106.7 g of chloropropyltrichlorosilane and 193.6 g of propyltrichlorosilane are added as a mixture to a 1 liter capacity three-necked flask equipped with stirrer, cooler and nitrogen inlet tube. A solution of 380 ml of ethanol and 17.2 g of water is added dropwise within 50 minutes while cooling. The reaction mixture is then heated for 4 hours under reflux and the hydrogen chloride gas is expelled. 228.0 g of a clear, pale yellow liquid are obtained after removing the excess ethanol. The ratio of propyl radical to chloropropyl radical is 2 to 1.
[0050] .sup.1H-NMR (CDCl.sub.3): .delta. 0.60 (m, 4H, Si--CH.sub.2--CH.sub.2--CH.sub.3), 0.75 (m, 2H, Si--CH.sub.2--CH.sub.2--C-H.sub.2--Cl), 0.95 (t, 6H, .sup.3J.sub.H-H=7 Hz, Si--CH.sub.2--CH.sub.2--C-H.sub.3), 1.20 (m, 12H, CH.sub.3--CH.sub.2--O--Si), 1.45 (m, 4H, Si--CH.sub.2--CH.sub.2--CH.sub.3), 1.85 (m, 2H, Si--CH.sub.2--CH.sub.2--C-H.s...
example 2
Reaction of a Chloropropyltrichlorosilane-Octyltrichlorosilane Mixture with Water and Ethanol
[0051] 106.7 g of chloropropyltrichlorosilane and 111.8 g of octyltrichlorosilane are added as a mixture to a 1 liter capacity three-necked flask equipped with stirrer, cooler and nitrogen inlet tube. A solution of 240 ml of ethanol and 10.7 g of water is added dropwise within 40 minutes while cooling. The reaction mixture is then heated for 4 hours under reflux and the hydrogen chloride gas is expelled. 182.3 g of a clear, colorless liquid are obtained after removing the excess ethanol. The ratio of octyl radicals to chloropropyl radicals is 1 to 1.
[0052] .sub.1H-NMR (CDCl.sub.3): .delta. 0.60 (m, 2H, Si--CH.sub.2--(CH.sub.2).sub.6--CH.sub.3), 0.80 (m, 2H, Si--CH.sub.2--CH.sub.2--CH.sub.2--Cl), 0.90 (t, 3H, .sup.3J.sub.H-H=7 Hz, Si--CH.sub.2--(CH.sub.2).sub.6--CH.sub.3), 1.25 (m, 9H, CH.sub.3--CH.sub.2--O--Si), 1.3-1.5 (m, 12H, Si--CH.sub.2--(CH.sub.2).sub-.6--CH.sub.3), 1.85 (m, 2H, Si--CH...
example 3
Reaction of a Chloropropyltrichlorosilane-Hexadecyltrichlorosilane Mixture with Water and Ethanol
[0053] 106.7 g of chloropropyltrichlorosilane and 74.1 g of hexadecyltrichlorosilane are added as a mixture to a 1 liter capacity three-necked flask equipped with stirrer, cooler and nitrogen inlet tube. A solution of 180 ml of ethanol and 8.0 g of water is added dropwise within 50 minutes while cooling. The reaction mixture is then heated for 4 hours under reflux and the hydrogen chloride gas is expelled. 145.3 g of a clear, pale yellow liquid are obtained after removing the excess ethanol. The ratio of hexadecyl radicals to chloropropyl radicals is 1 to 2.5.
[0054] .sup.1H-NMR (CDCl.sub.3): .delta. 0.65 (m, 2H, Si--CH.sub.2--(CH.sub.2).sub.14--CH.sub.3), 0.80 (m, 5H, Si--CH.sub.2--CH.sub.2--CH.sub.2--Cl), 0.85 (t, 3H, .sup.3J.sub.H-H=7 Hz, Si--CH.sub.2--(CH.sub.2).sub.14--CH.sub.3), 1.25 (m, 15H, CH.sub.3--CH.sub.2--O--Si), 1.3-1.5 (m, 28H, Si--CH.sub.2--(CH.sub.2).sub-.14--CH.sub.3), 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com