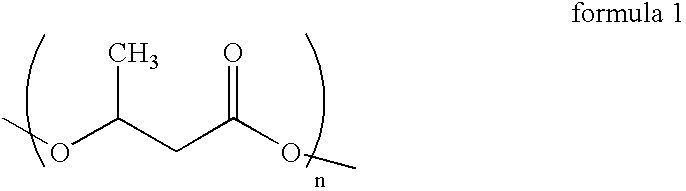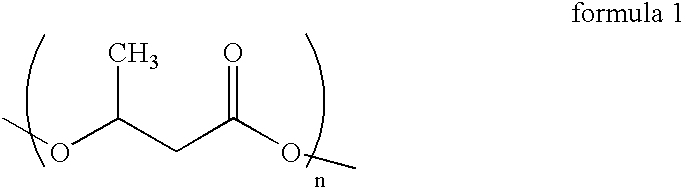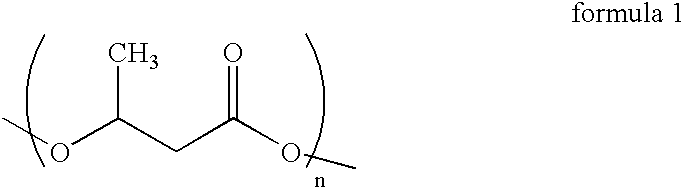Process for the isolation of polyhydroxybutyrate from Bacillus mycoides RLJ B-017
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0053] Isolation
[0054] Activated sludge sample, from wastewater treatment plant was appropriately diluted and then spread on YPM-agar plates containing (g 1.sup.-1): yeast extract, 10; bactopeptone, 10; meat extract, 5; NaCl, 5; and agar, 20. The plates were incubated at 30.degree. C. for 72 h. Several colonies appeared on the plates and were screened for their PHB production. Isolate RLJ B-017, thus selected was used in this study.
[0055] Identification of the isolated strain
[0056] Morphological and taxonomic features of the selected isolate were examined by the established method (Sheath et. Al, 1986). The results are as shown Table 1 in Example 1.
[0057] Organism and Maintenance
[0058] Bacillus mycoides RLJ B-017, was grown at 30.+-.0.5.degree. C. and maintained at 4.degree. C. periodical transfer on YPM-agar slants.
[0059] Inoculum Preparation
[0060] The inoculum was grown on nutrient rich medium occupying 20% of the flask volume. The nutrient rich medium consists of (g 1.sup.-1): su...
example-3
[0067] Isolation
[0068] Activated sludge sample, from wastewater-treatment plant was appropriately diluted and then spread on YPM-agar plates containing (g 1-1): yeast extract, 10; bactopeptone, 10; meat extract, 5; and agar, 20. The plates were incubated at 30.degree. C. for 72 h. Several colonies appeared on the plates were screened for their PHB production. Isolate RLJ B-017, thus selected was used in this study.
[0069] Identification of the isolated strain
[0070] Morphological and taxonomic features of the selected isolate was examined by the established method (Sheath et. Al, 1986) The results are as shown Table 1 in example 1.
[0071] Organism and Maintenance
[0072] Bacillus mycoides RLJ B-017, was grown at 30.+-.0.5.degree. C. and maintained at 4.degree. C. by periodical transfer on YPM-agar slants.
[0073] Inoculum Preparation
[0074] The inoculum was grown on nutrient rich medium occupying 20% of the flask volume. The nutrient rich medium consists of (g 1.sup.-1): sucrose, 20; nutrie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com