Method for preparing and using polyoxyethylated castor oil in pharmaceutical compositions
a technology of polyoxyethylated castor oil and composition, which is applied in the directions of drug compositions, biocide, plant/algae/fungi/lichens ingredients, etc., can solve the problems of drug precipitation, poor water soluble paclitaxel, and unstable injection compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
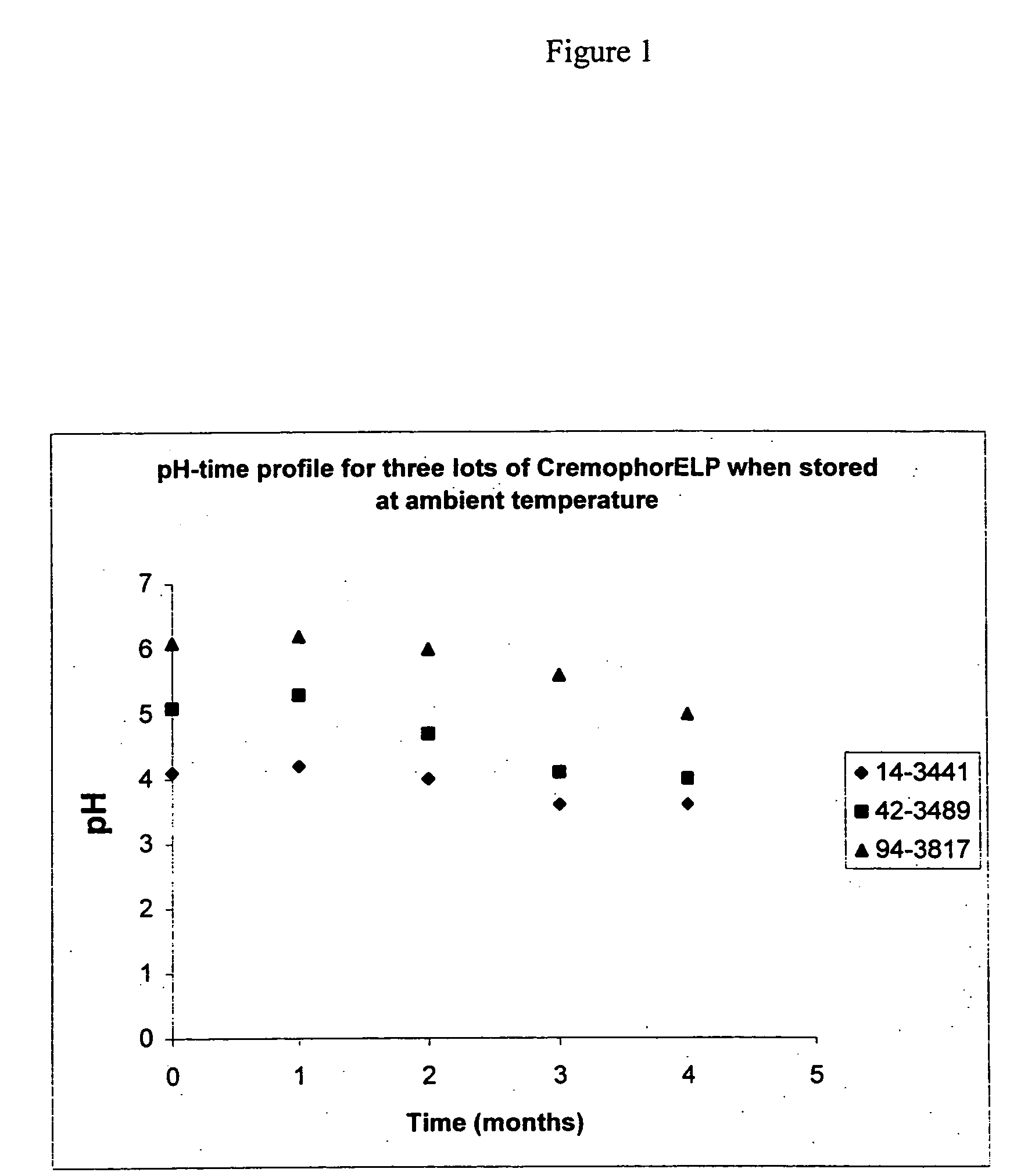
[0090] Three different lots of Cremophor.RTM. ELP were stored in original plastic containers under ambient conditions. Samples from these lots were taken at different times. The pH of these samples, after being diluted 1:10 with water, was measured. Table 1 provides the pH measurements for the samples. The pH measurements are also plotted in FIG. 1. As can be seen from both Table 1 and FIG. 1, Cremophor.RTM. ELP becomes more acidic over time. Without being bound by theory, it appears from FIG. 1 that the acidification of the Cremophor.RTM. ELP decelerates over time under ambient conditions such that the resulting pH may plateau around pH 3.6 and 4.0.
1TABLE 1 pH of different lots of Cremophor ELP measured at predetermined time intervals. Lot # 14-3441 42-3489 94-3817 pH on Certificate of Analysis provided by BASF TTime (Months) 6.4 6.1 6.1 Initial 4.1 5.1 6.1 1 4.2 5.3 6.2 2 4.0 4.7 6.0 3 3.6 4.1 5.6 4 3.6 4.0 5.0
example 2
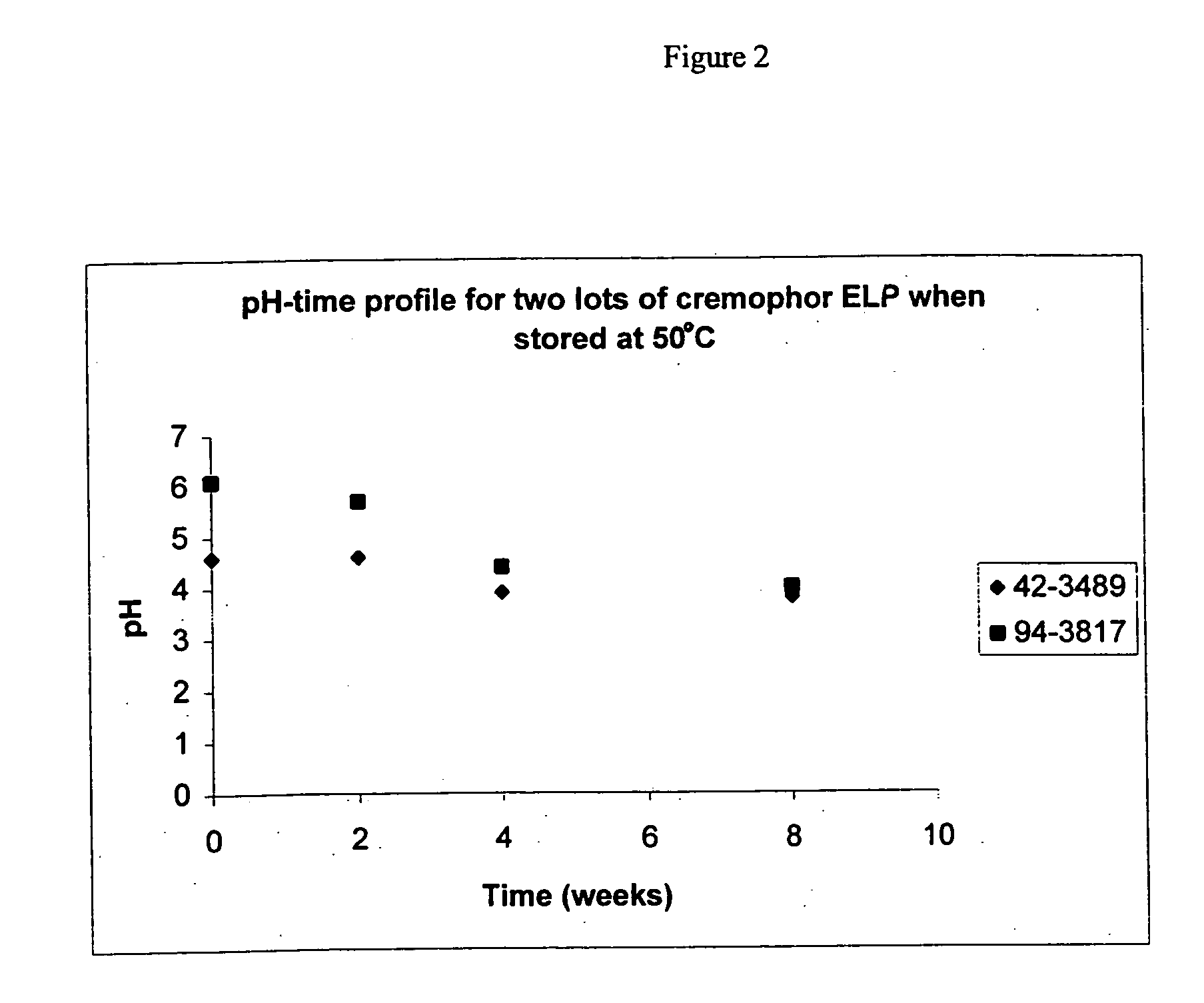
[0091] Twenty (20) mL aliquots obtained from two lots of Cremophor.RTM. ELP were stored in glass vials at 50.degree. C. Samples from these aliquots were taken at different times. The pH of these samples, after being diluted 1:10 with water, was measured. Table 2 provides the pH measurements for the samples. The pH measurements are also plotted in FIG. 2.
[0092] As can be seen from both the table and FIG. 2, increasing the storage temperature from ambient to 50.degree. C. accelerates the rate at which the acidity of the Cremophor increases. For example, the pH of samples from Lot 94-3817 stored at 50.degree. C. dropped from 6.1 to 4.4 in four weeks whereas it took samples from Lot 94-3817 stored at ambient conditions to drop from pH 6.1 to pH 5.0 in four months (see Table 1 for results).
2TABLE 2 pH measurements of different lots of Cremophor .RTM. ELP at predetermined time intervals following storage at 50.degree. C. 42-3489 94-3817 pH on Certificate of Analysis provided by BASF Time ...
example 3
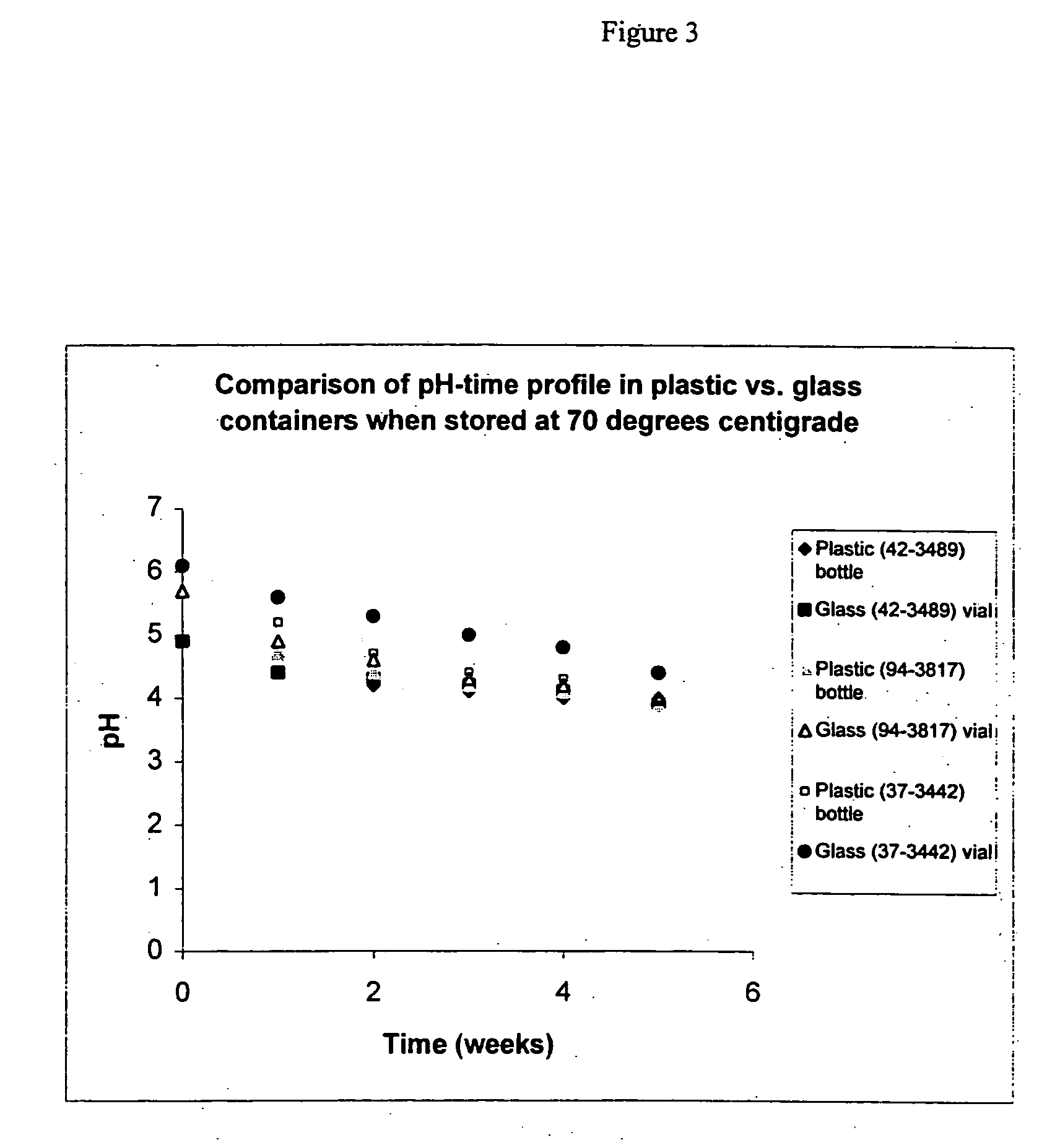
[0093] Twenty (20) mL aliquots obtained from three lots of Cremophor.RTM. ELP were stored in glass vials and in plastic containers at 70.degree. C. Samples from these aliquots were taken at different times. The pH of these samples, after being diluted 1:10 with water, was measured. Table 3 provides the pH measurements for the samples. The pH measurements are also plotted in FIG. 3.
[0094] Increasing the storage temperature from 50.degree. C. to 70 .degree. C. does not greatly accelerate the rate at which the acidity of the Cremophor increases when results for different lots of Cremophor ELP in Table 2 are compared with corresponding results in Table 3.
[0095] The type of container used to age Cremophor.RTM. ELP did not appear to be critical. For example, the pH of samples from Lot 94-3817 stored at 70.degree. C. dropped from pH 6.1 to 4.1 in four weeks in plastic container and pH 4.2 in the same time when stored in glass container. However, the pH data from Lot #37-3442 suggests some ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com