Mucin peptide with immunoenhancing properties
a peptide and immunoenhancing technology, applied in the field of peptides with immunoenhancing properties, can solve problems such as biocompatibility with formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
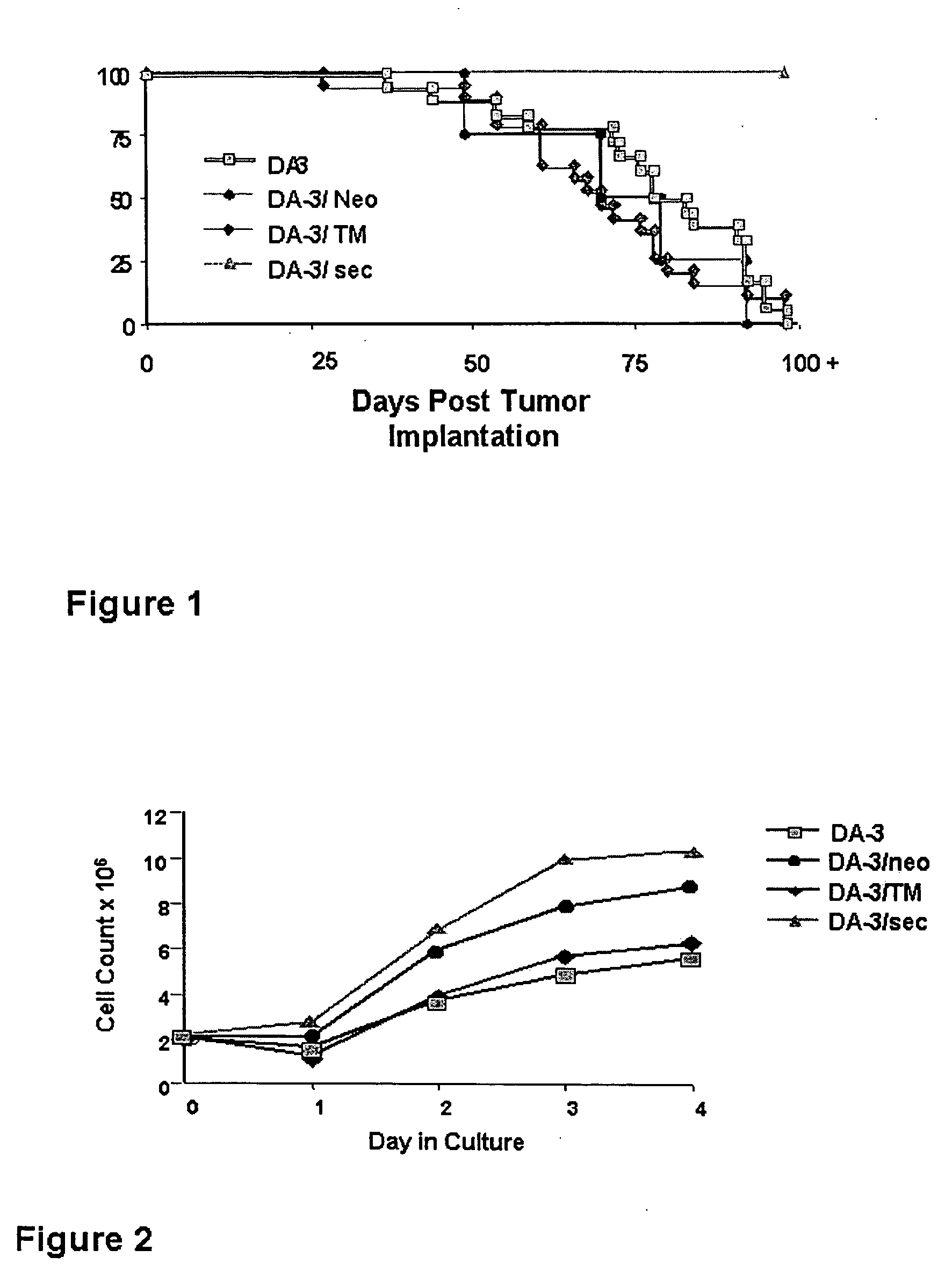
[0056] DA-3 mammary tumor cells were transfected as described in Materials and Methods with either the transmembrane and secretory isoforms of the human MUC1. The success of the transfections was proven by staining the cells for the presence of the MUC1 tandem repeat using the H23 antibody specific for this sequence (5). The resulting cell lines were used in in vivo experiments to determine the incidence and time of tumor appearance in BALB / c mice. As seen in Table 1, implantation of DA-3, DA-3 / neo and DA-3 / TM mammary tumor cells into mice gave rise to palpable tumors of approximately the same size by seven days and by 15 days essentially all animals had sizable tumors.
TABLE 1Incidence and Time of Tumor Appearance in BALB / c MiceTumorDay of Tumor appearanceType715253712+ monthsDA-332 / 3838 / 38DA-3 / neo17 / 2524 / 2525 / 25DA-3 / TM29 / 3633 / 3635 / 3636 / 36DA-3 / sec 0 / 70 0 / 70 1 / 70 1 / 701 / 70
Surprisingly, the DA-3 cells transfected with the MUC1 secreted form (DA-3 / sec) failed to cause tumor developme...
example 2
[0057] A trivial explanation to these results could be that the transfection process had selectively impaired the basic growth potential of the DA-3 / sec cells. To test this possibility we investigated the in vitro growth characteristics of the four types of DA-3 tumor cells. As shown in FIG. 2, the parent cell line and all the various transfectants grew with similar kinetics in vitro. In fact, the DA-3 / sec cells seemed to proliferate better than the other cell lines, indicating that the in vitro growth potential of these cells has not been altered by the transfection manipulations.
example 3
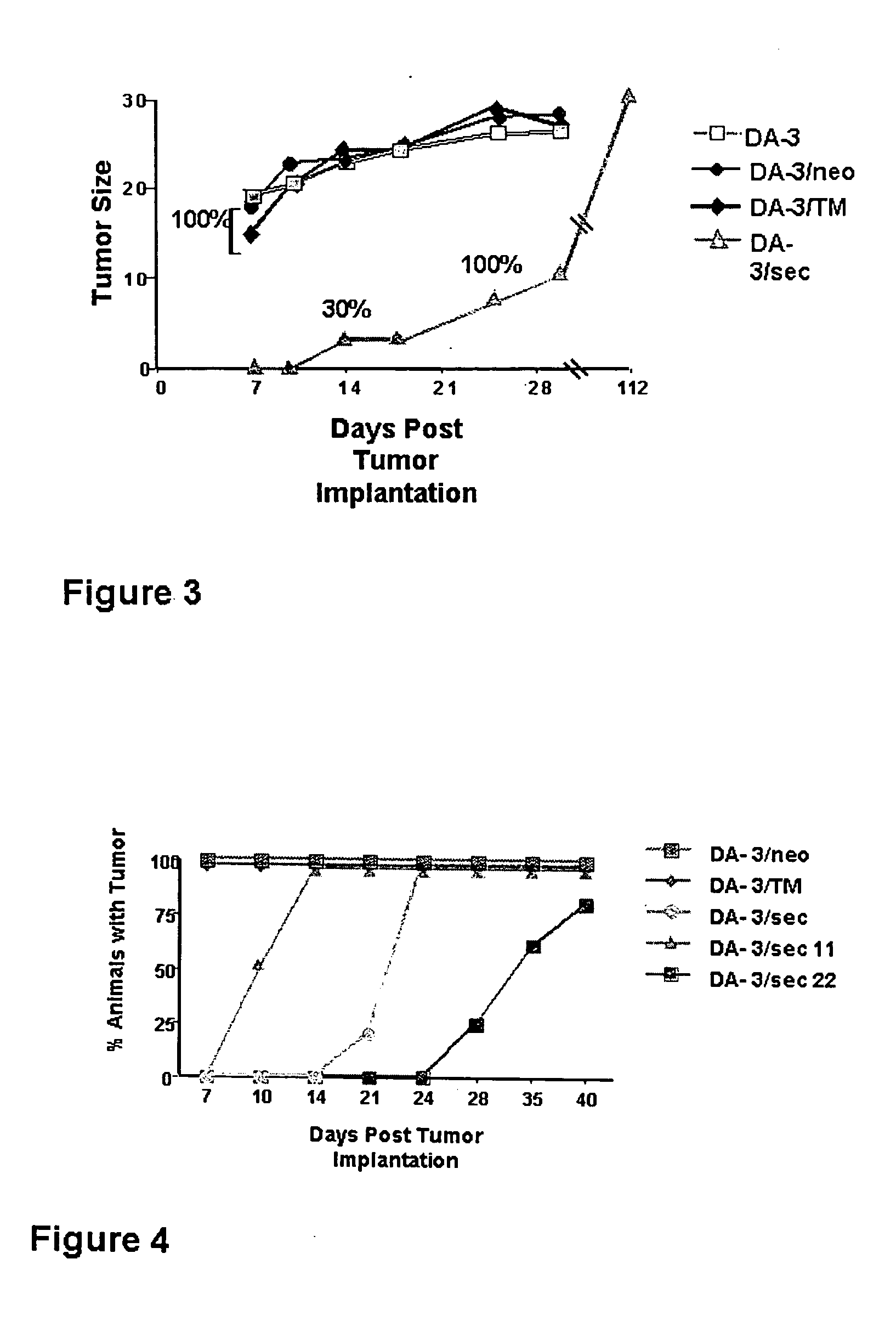
[0058] In order to determine whether the DA-3 / sec cells had lost all tumorigenic potential in vivo, all four DA-3 cell lines were implanted in nu+ / nu+BALB / c mice and the incidence of tumor appearance and tumor size at various times were assessed. FIG. 3 shows that the DA-3, DA-3 / neo, and DA-3 / TM cells cause palpable tumors by seven days after implantation in BALB / c nude mice and they grew in a manner similar to that of intact BALB / c animals. In contrast with the results in Table 1 and FIG. 1, DA-3 / sec tumor cells gave rise to tumors in 30% of all nude mice by 14 days and by 25 days all these animals had tumors. Thus, the lack of tumor growth in the intact BALB / c mice implanted with the DA-3 / sec cells appears to be immunologically controlled, since implantation of this tumor in nude mice resulted in 100% tumor takes, albeit at a slower time of appearance. These results were repeated using other two DA-3 tumor cells separately transfected with the expression plasmid harboring the secr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| biocompatible | aaaaa | aaaaa |
| TM | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com