Protamine fragment compositions and methods of use
a technology of protamine fragments and compositions, applied in the bioactive, low-toxicity fragments of protamine, protamine fragments, etc., can solve the problems of insufficient length to bind thrombin, high incidence of bleeding complications, lack of appropriate clinical antidote to combat the potential risk of induced bleeding, etc., to prolong the adsorption of insulin, reduce post-operative bleeding, and reduce immunogenicity and/or antigenicity and/or toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Low Molecular Weight Protamine
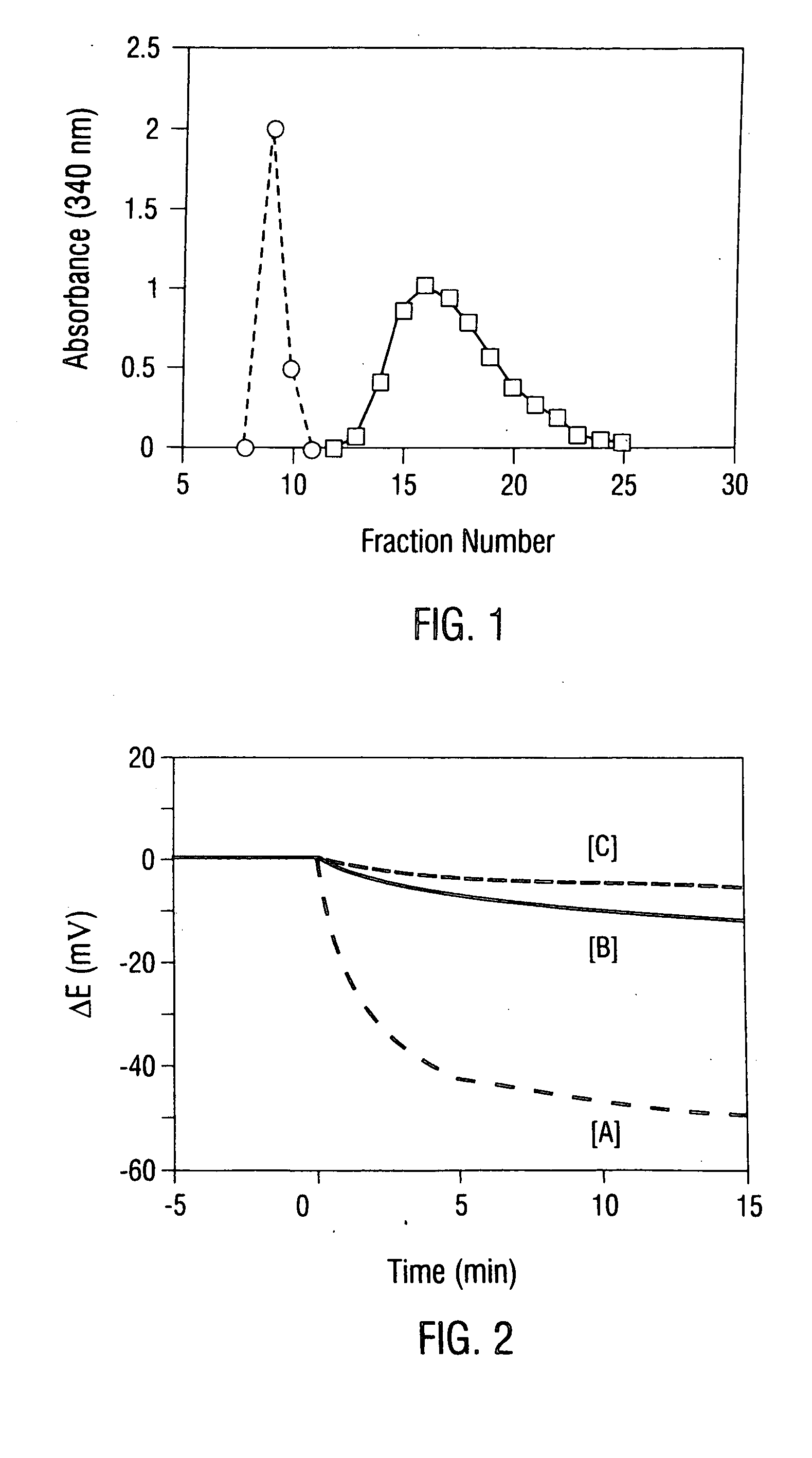
[0134] In this example, low molecular weight protamine (LMWP) fragments were derived from native protamine by enzymatic digestion of protamine with thermolysin. Then, the heparin-neutralizing ability of the LMWP fragments was examined in vitro, using a electrochemical sensor method and various biological assays. Next, the immunogenicity and antigenicity of the LMWP species were examined in mice, using an appropriately selected enzyme-linked immunosorbent assay (ELISA). The enzymatic digestion of protamine yields LMWP that contained anti-heparin activity but lacked antigenicity and immunogenicity.
A. Materials and Methods
[0135] Protamine sulfate (Clupeine from herring), 2,4,6-trinitrobenzene sulfonic acid (TNBS), thermolysin (EC 3.4.24.4), Freund's adjuvant, and goat-antimouse-IgG-alkaline phosphatase were purchased from Sigma Chemical Co. (St. Louis, Mo.). Porcine intestine heparin (169 IU / mg; average molecular weight of 13,000 daltons), antithrombin...
example 2
Heparin Neutralization and Complement Activation by LMWP
A. Neutralization of Heparin / LMWH
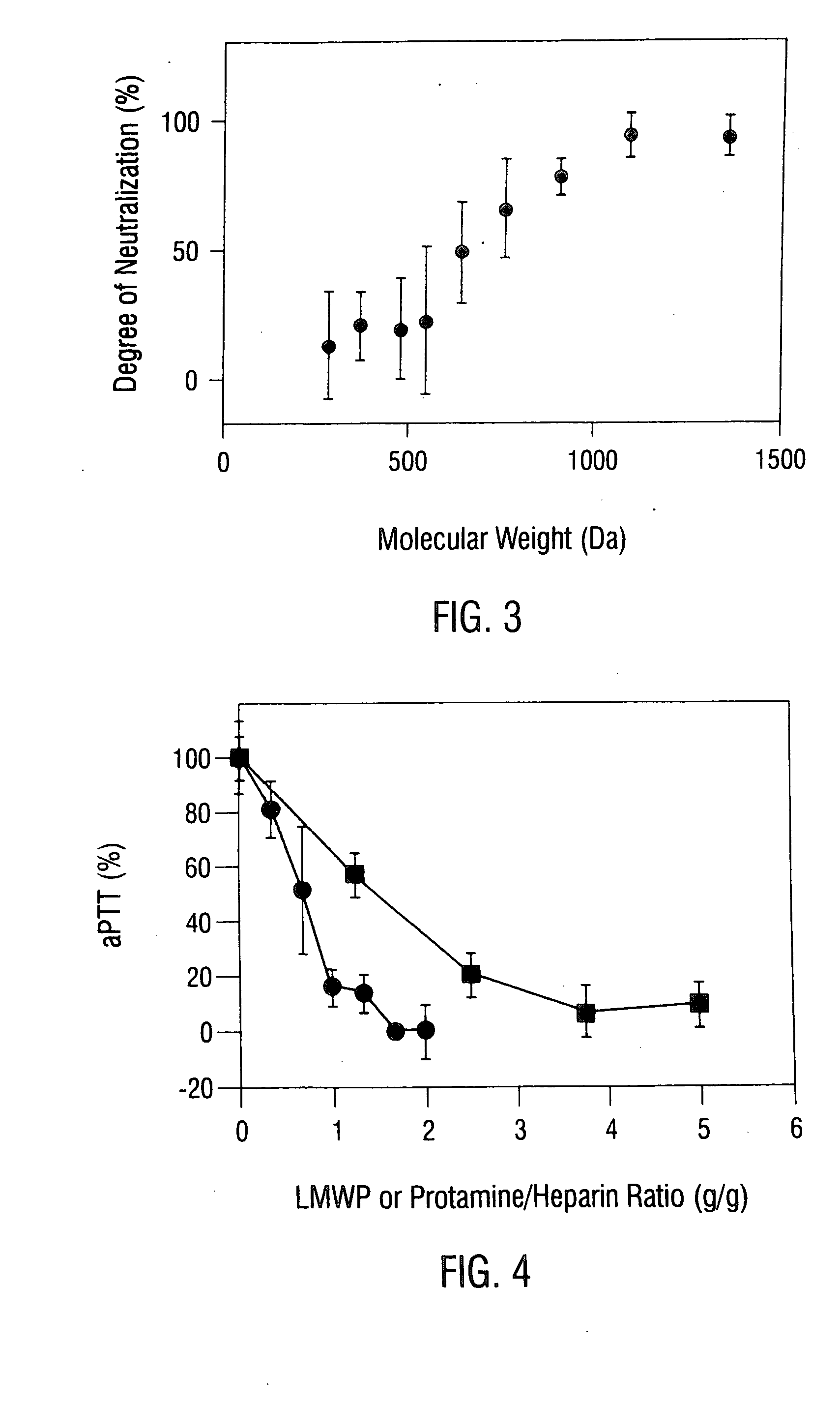
[0152] Neutralization of the anticoagulant functions of both heparin and LMWH by protamine and LMWP is studied in human plasma using the aPTT clotting assay and the chromagenic anti-Xa and anti-IIa assays (Yang et al., 1986). The aPTT values of the test samples are measured using a fibrometer (Fibrosystem; Becton-Dickinson, Cockeysville, Md.), and the anti-Xa and anti-IIa activities are measured using the chromagenic substrates S-2222 and S-2238, respectively. Unless otherwise stated, heparin powder (MW ˜15,000 Da; Anticoagulant activity: 167 IU / mg; Anti-Xa activity: 110 IU / mg; Anti-IIa activity: 85 IU / mg) from Pharmacia-Hepar (Franklin, Ohio) and Enoxaparin powder (MW ˜4400 Da; Anticoagulant activity: 32 IU / mg; Anti-Xa activity: 96 IU / mg; Anti-IIa activity: 27 IU / mg) by Rhone-Poulenc Rorer (Collegeville, Pa.) are used for these studies. The neutralizing activity of LMWP towards heparin or LM...
example 3
Combination of LMWP With Insulin
[0169] Among the four groups of patients at high risk to protamine responses, the diabetic patients represent the largest population. Since the underlying cause for the risk is related to the immunogenicity of the administered commercial protamine, the use of LMWP, if proven to be less immunogenic, for insulin formulation would be of significant benefit to this group of patients. To examine this possibility, LMWP was conjugated with insulin and then tested in diabetic rats for the control of blood glucose levels.
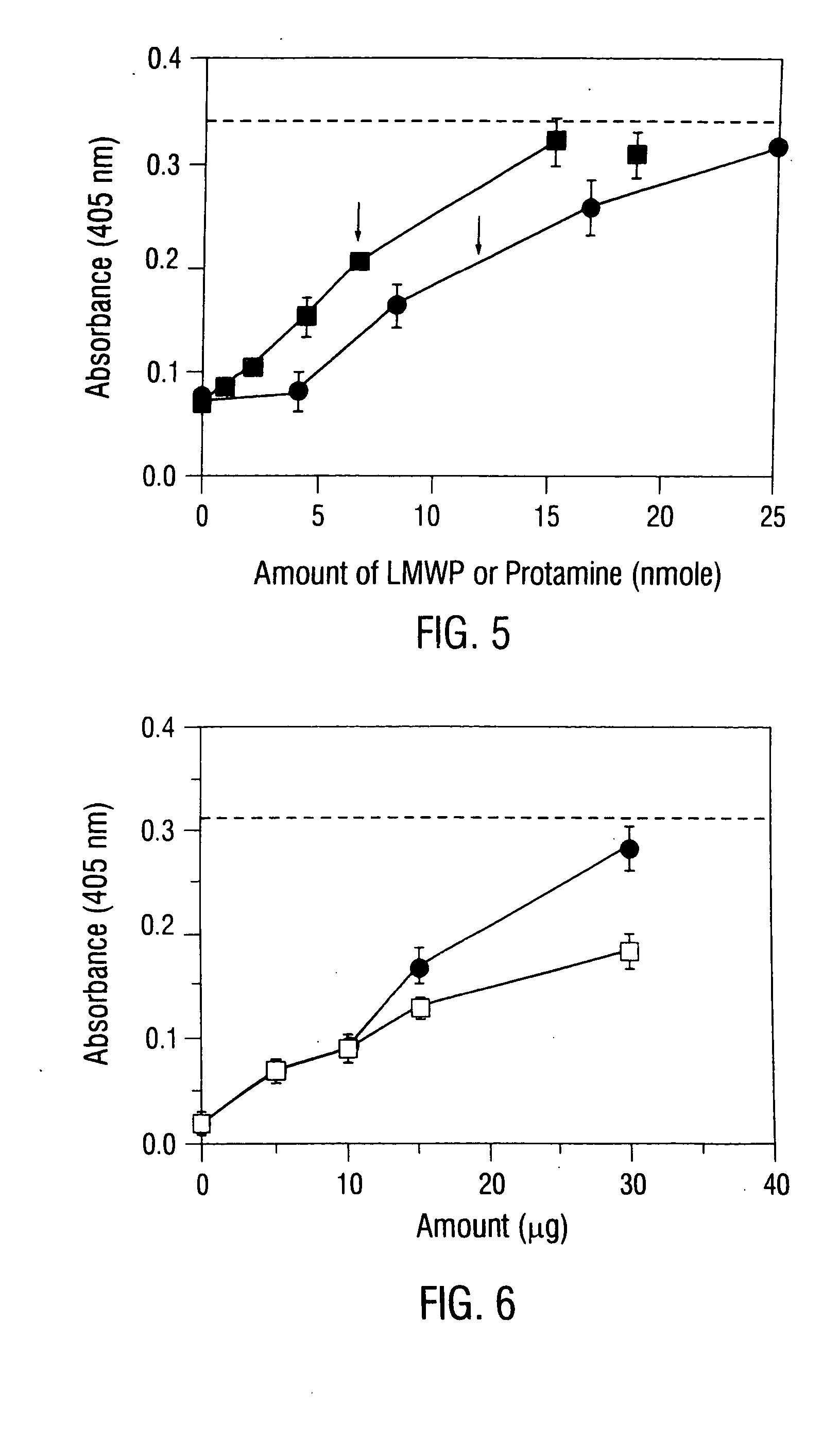
[0170] Protamine prolonged insulin's adsorption by the formation of a complex (PZI or NPH) that is not soluble at physiological pH (the complex has a pI of 7.4). To examine whether LMWP provides the same function, insulin (80 USP U / ml) and LMWP (1 mg / mL) solutions were combined at pH 7.4 in different volume ratios, followed by measuring the turbidity of these solutions at 350 nm. The volume ratio of 1:8 of insulin:protamine yielded the highe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com