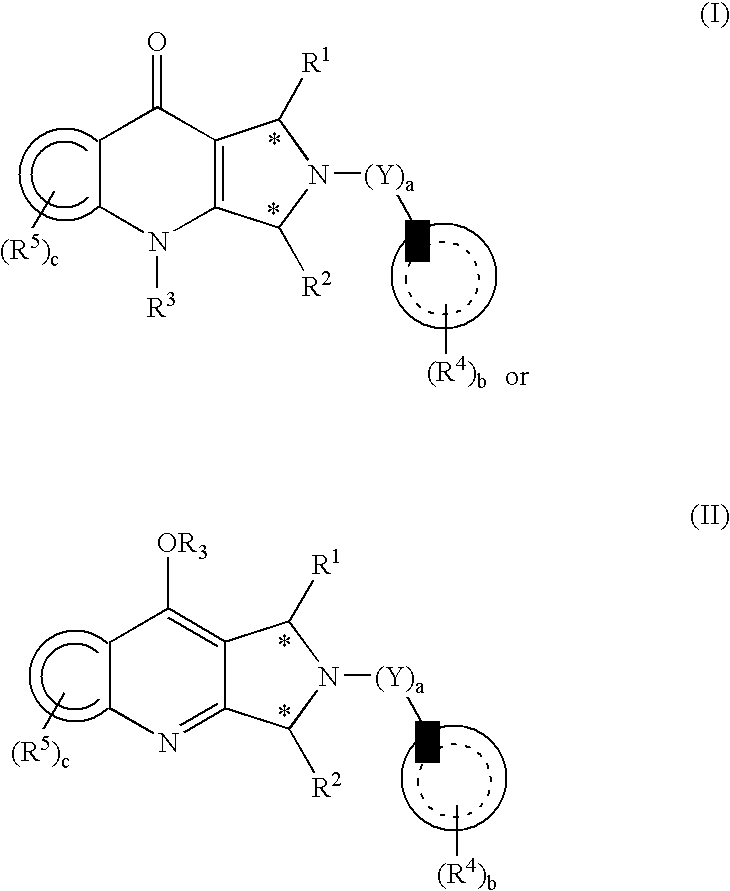
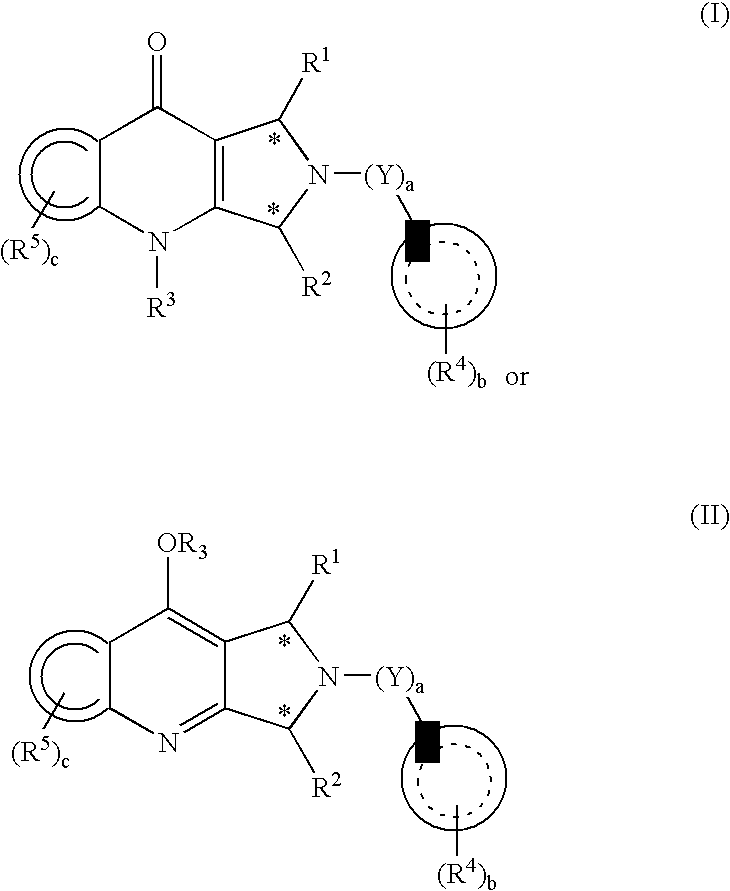
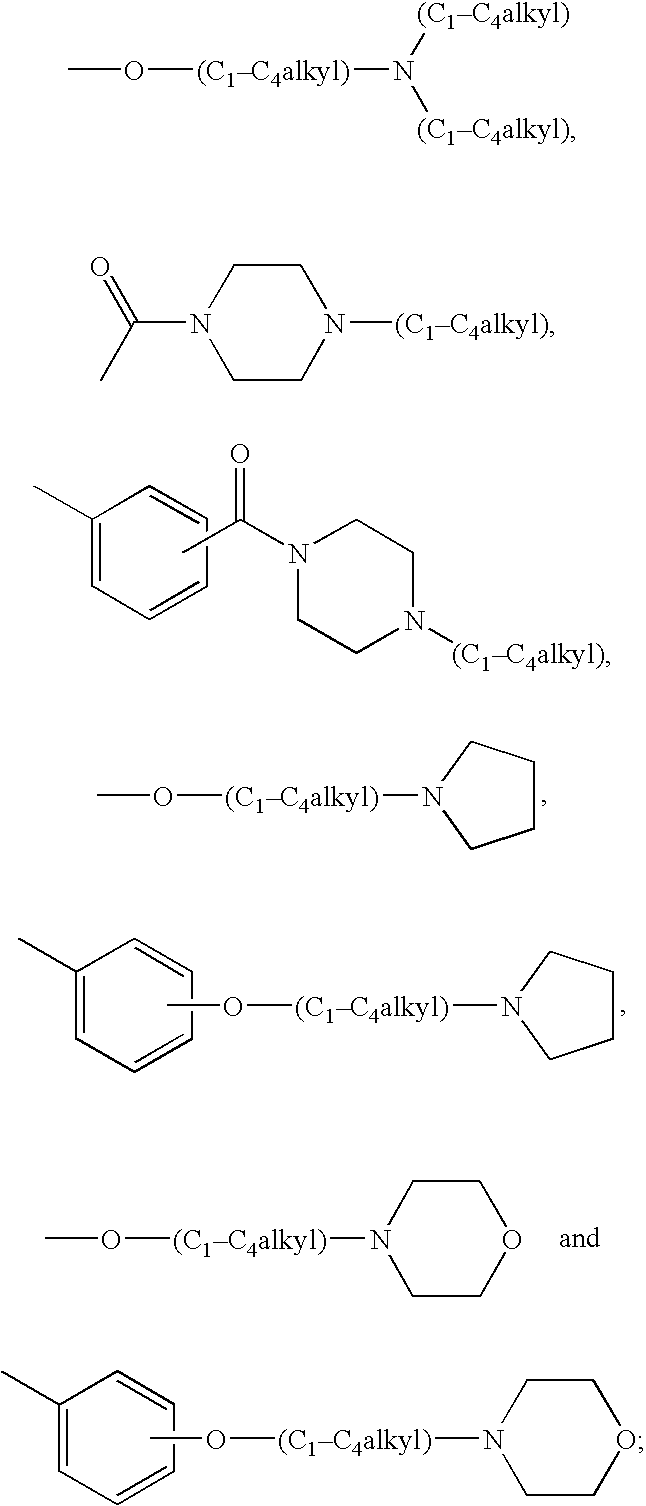
Substituted pyrrolopyridinone derivatives useful as phosphodiesterase inhibitors
a technology of pyrrolopyridinone and derivatives, which is applied in the field of substituted pyrrolopyridinone derivatives, can solve the problems of ed, side effects, fibrosis, etc., and achieve the effects of reducing the risk of ed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-(3,4-Methylenedioxyphenyl)-2-benzyl-2,3,4,9-tetrahydro-1H-β-carboline
[0134] To a solution of the 1-(3,4-methylenedioxyphenyl)-2,3,4,9-tetrahydro-1H-β-O-carboline (prepared according to the process as disclosed in WO97 / 43287, Intermediate 7, page 24) (7.37 g, 25 mmol) in dry DMF (25 mL) was added triethylamine (3.52 mL, 25 mmol) and benzyl bromide (3.00 mL, 25 mmol). The mixture was stirred at ambient temperature overnight and added dropwise to a solution of sodium hydroxide (25 mmol) in water (200 mL). A precipitate was formed, collected by vacuum filtration, washed with water (2×50 mL), and dried in vacuo overnight to yield the product as a freely flowing pale yellow powder.
[0135] MS (m / z) 383 (MH+)
[0136]1H NMR (CDCl3) δ 2.57-2.89 (series of m, 3H), 3.18-3.23 (m, 1H), 3.33 (d, J=13.7 Hz, 1H), 3.63 (d, J=13.7 Hz, 1H), 4.55 (s, 1H), 5.94 (nd, J=2.2 Hz, 2H), 6.77-7.52 (series of m, 13H).
example 1a
(R)-1-(3,4-Methylenedioxyphenyl)-2-benzyl-2,3,4,9-tetrahydro-1H-β-carboline
[0137] Following the procedure as described in Example 1, (R)-1-(3,4,-methylenedioxyphenyl)-2,3,4,9-tetrahydro-1H-β-carboline was reacted to produce the title compound.
[0138] MS (m / z) 383 (MH+)
example 2
1-(2,3-Dihydrobenzofuran-5-yl)-2-benzyl-2,3,4,9-tetrahydro-1H-β-carboline
[0139] The title product was prepared according to the process described in Example 1 using 1-(2,3-dihydrobenzofuran-5-yl)-2,3,4,9-tetrahydro-1H-β-carboline as the starting reagent.
[0140] MS (m / z) 381 (MH+)
[0141]1H NMR (CDCl3) δ 2.59-2.90 (series of m, 3H), 3.13-3.24 (m, 3H), 3.33 (d, J=13.5 Hz, 1H), 3.93 (d, J=13.5 Hz, 1H), 4.56 (t, J=8.6 Hz, 2H), 6.75 (d, J=8.1 Hz, 1H), 7.05-7.35 (series of m, 10H), 7.49-7.52 (m, 1H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com