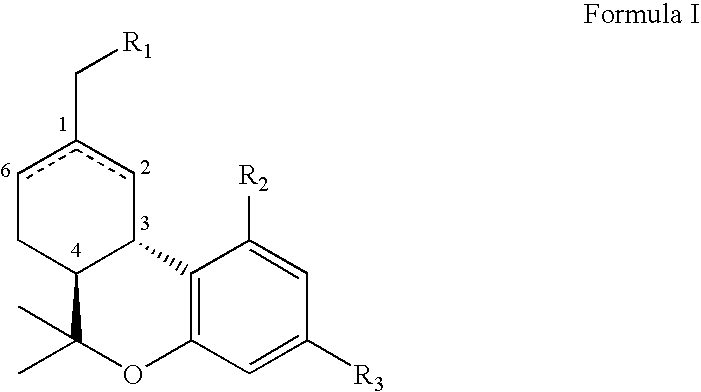
Dexanabinol and dexanabinol analogs regulate inflammation related genes
a technology of dexanabinol and dexanabinol, which is applied in the field of pharmaceutical compositions, can solve the problems of pharmacological treatment, unclear mechanisms underlying some therapeutic effects of cannabinoid derivatives, and inability to detect and treat inflammation, so as to prevent, relieve or treat patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
RNA Preparation and Real-time RT-PCR
[0140] Total RNA was prepared using SV total RNA isolation system (Promega). The cells or tissues were homogenized in lysis buffer. The lysates were transferred to an RNA isolation column, treated with DNAse, washed and eluted according to kit instructions. RNA concentrations were determined using GeneQuant II (Phamacia-Amersham). Complementary DNA (cDNA) was synthesized from total RNA using SUPERSCRIPT II reverse transcriptase (Life Technologies). 2 μg of total RNA were combined with an oligo (dT)15 primer, 0.5 mM dNTP mix, 8 units of reverse transcriptase and other reaction components up to a final volume of 20 μl according to the kit instructions. The reaction mixture was incubated at 42° C. for 45 min and inactivated at 70° C. for 15 minutes. Quantitative real-time RT-PCR includes 1 μl of the cDNA, 300 nM of the appropriate forward and reverse primers (see below) and 7.5 μl of the reaction mix containing buffer, nucleotides, Taq polymerase an...
example 2
Quantitation of Protein Using ELISA
[0142] The technique used to quantify the amount of a given protein in a liquid sample, either tissue culture supernatant or body fluid, is based on Enzyme Linked ImmunoSorbent Assay (ELISA) methodology. Either commercially available or established in house, the assay is based on the capture of the protein of interest by specific antibodies bound to the bottom of an ELISA plate well. Unbound material is washed away, the captured protein is then exposed to a secondary antibody generally labeled with horseradish peroxidase (HRP) or alkaline phosphatase (ALP). Again the unbound material is washed away, the samples are then incubated with the appropriate substrate yielding a colorimetric reaction. The reaction is stopped and reading is carried out in a spectrophotometer at the appropriate wavelength. Samples are tested at least in duplicate and the appropriate standard curve, consisting of serial dilutions of the recombinant target protein, is incorpo...
example 3
Quantitation of COX-2 Gene Expression in LPS Activated Macrophages and LPS Injected Mice Brains
[0143] We previously showed that dexanabinol and its analogs reduce the levels of secreted PGE2 in LPS activated mouse macrophages (RAW 264.7) cells in vitro (WO 01 / 98289). The IC50 for inhibition of PGE2 secretion were determined and found to be 10 μM, 10 μM, 4 μM, and 8 μM, for dexanabinol, PRS-211,092, PRS-211,095 and PRS-211,220, respectively. For comparison, the IC50 for inhibition of PGE2 secretion for the known anti-inflammatory drugs Celecoxib, Rofecoxib and NS-398 were respectively 5 nM, 100 nM and 100 nM in the same experimental setup. We assumed then that this phenomenon observed at the level of PGE2 secretion was due to direct inhibition of COX-2 enzymatic activity. To check this hypothesis we have now tested in vitro the enzymatic activity of recombinant COX-2 on its substrate in the presence of dexanabinol and its analogs PRS-211,092, PRS-211,095 and PRS-211,220. The assay r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com