Mesothelin vaccines and model systems
a vaccine and model technology, applied in the field of cancer prognosis, cancer prognosis, and anticancer drug development, can solve the problems of inability to generate patient-derived t cell lines and clones that can be employed to identify immune relevant tumor targets, and the response to specific human tumor antigens has not yet been correlated, so as to achieve the effect of longer survival tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0094] To identify genes that can serve as potential immune targets for the majority of pancreatic adenocarcinoma patients, we focused only on those genes that were non-mutated, overexpressed by the majority of pancreatic cancer patients, and overexpressed by the vaccine cell lines. One gene at the top of this list was mesothelin (20, 21). For comparison and validation purposes we also looked at prostate stem cell antigen (PSCA). SAGE data demonstrated PSCA to be expressed by pancreatic tumors at similar levels to that of mesothelin (22).
[0095] We used the combination of two public use computer algorithms (23-25) to predict peptide nonamers that bind to three common human leukocyte antigen (HLA)-class I molecules. All 14 patients treated with the allogeneic GM-CSF vaccine express at least one of these HLA-Class I molecules (Table 2). The predictive algorithm “BIMAS”, ranks potential HLA binding epitopes according to the predictive half-time disassociation of peptide / HLA complexes (...
example 2
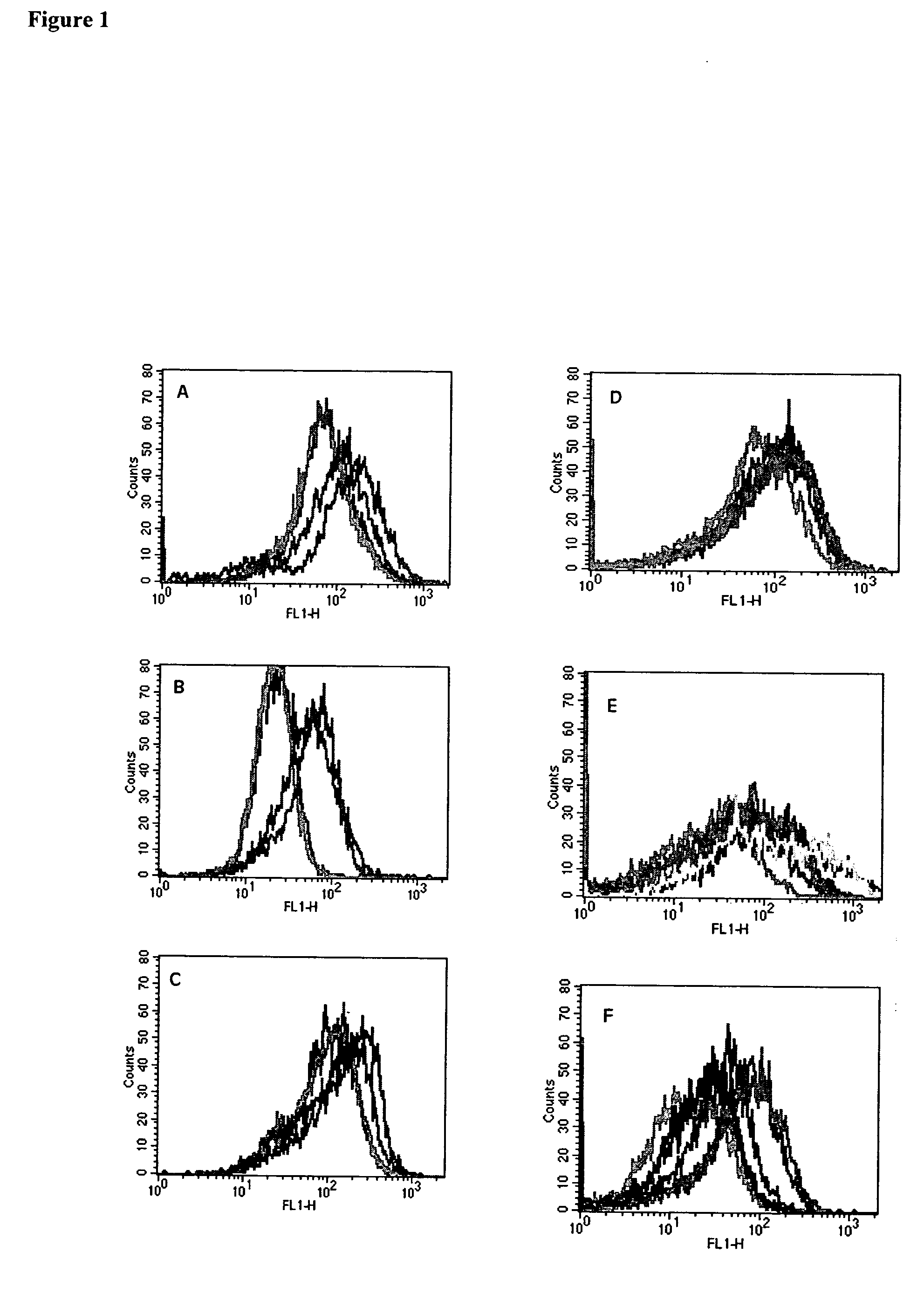
[0101] To determine if mesothelin and PSCA are recognized by CD8+ T cells, we screened antigen-pulsed T2 cells with CD8+ T cell enriched PBL from patients that have received an allogeneic GM-CSF secreting pancreatic tumor vaccine. We previously reported the association of in vivo post-vaccination delayed type hypersensitivity (DTH) responses to autologous tumor in three of eight patients receiving the highest two doses of vaccine. These “DTH responders” (each of whom had poor prognostic indicators at the time of primary surgical resection (27) are the only patients who remain clinically free of pancreatic cancer >4 years after diagnosis ((27), Table 2). PBL obtained prior to vaccination and 28 days after the first vaccination were initially analyzed. T2-A3 cells pulsed with the two A3 binding epitopes were incubated overnight with CD8+ T cell enriched lymphocytes isolated from the peripheral blood of patient 10 (non-DTH responder who relapsed 9 months after diagnosis) and 13 (DTH re...
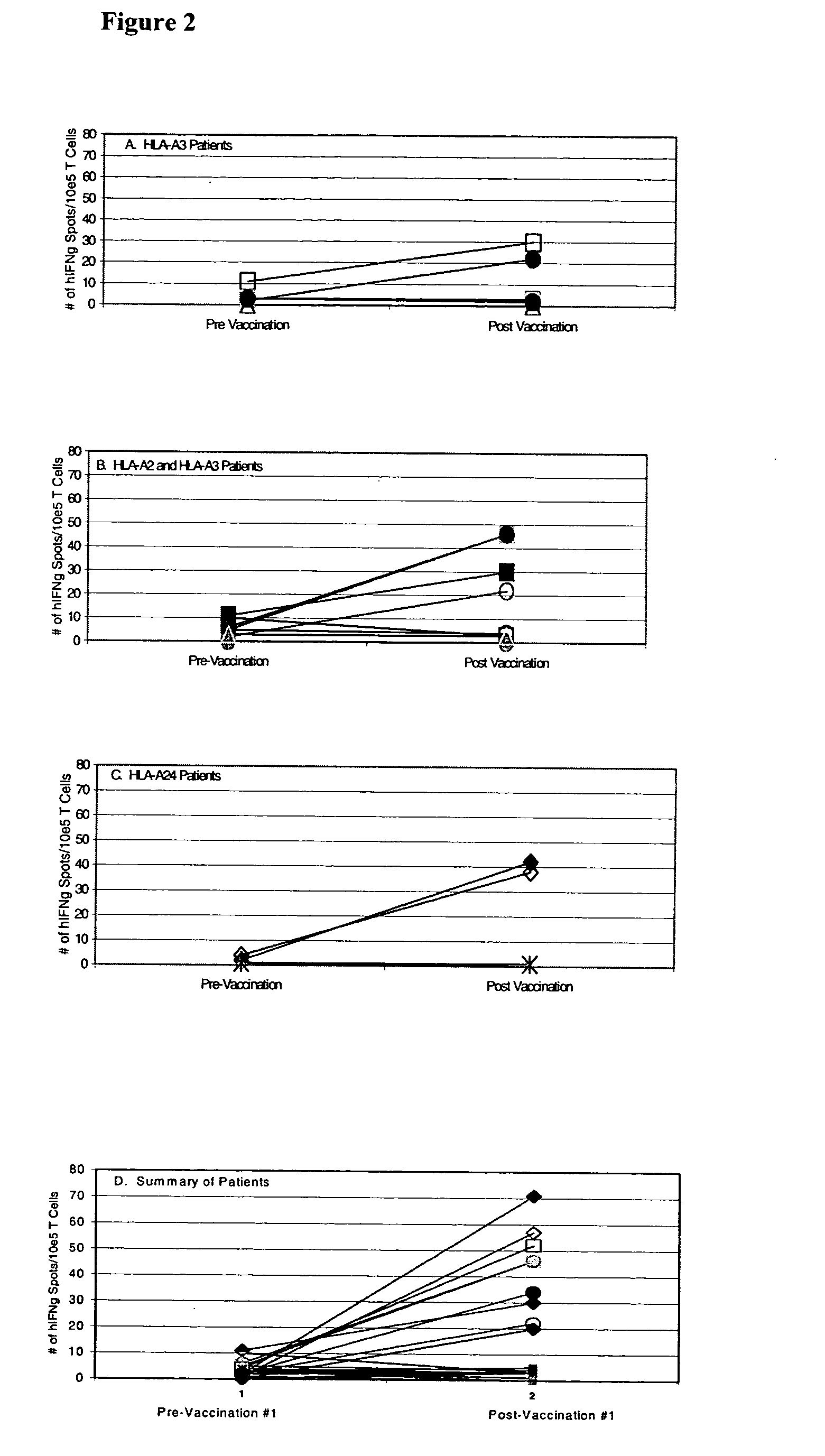
example 3
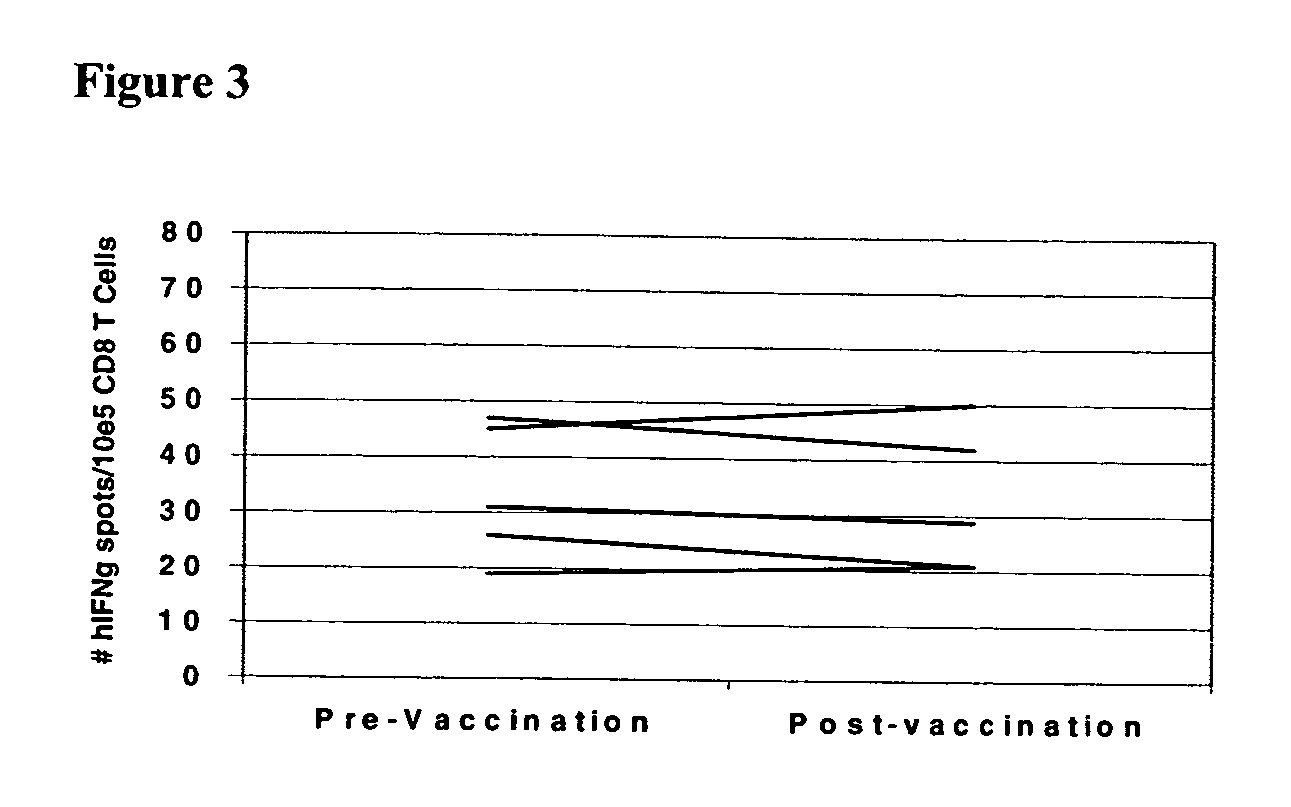
[0106] The above data clearly demonstrate a correlation of in vivo DTH response to autologous tumor and long term disease-free survival with the post-vaccination induction of mesothelin-specific CD8+ T cell responses. It is possible, however, that this correlation represents generalized immune suppression (in the patients who failed to demonstrate post-vaccination DTH responses to their autologous tumor and who had disease progression), rather than a vaccine specific induction of T cell responses to mesothelin in the DTH responder patients who remain disease-free. To demonstrate that the post-vaccination induction of mesothelin-specific CD8+ T cells is tumor antigen-specific, we evaluated each HLA-A2 positive patient for T cell responses to the HLA-A2-binding influenza matrix peptide, M1 (28). We chose the influenza M1 peptide because most patients on the vaccine study had received an influenza vaccine sometime prior to enrollment. As shown in FIG. 3, all HLA-A2 positive patients de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com