Process for the preparation of paroxetine substantially free of alkoxy impurities
a technology of paroxetine and impurities, which is applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of defluorination of intermediates, particularly problematic defluorination, and inability to effectively separate alkoxy impurities from paroxetine or its intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
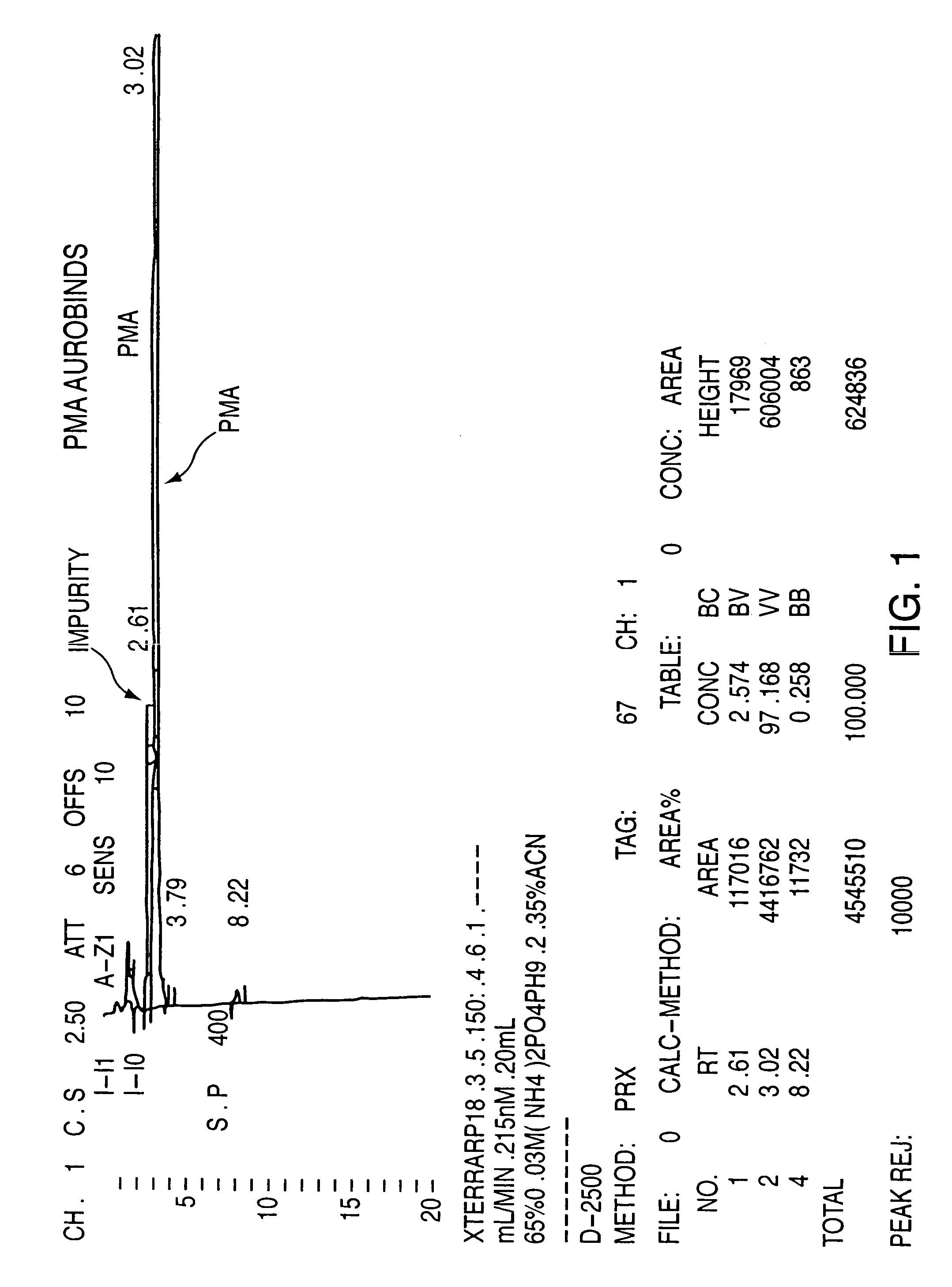
Purification of 1-methyl-3-hydroxymethyl-4-(4′-fluorophenyl)piperidine (PMA)
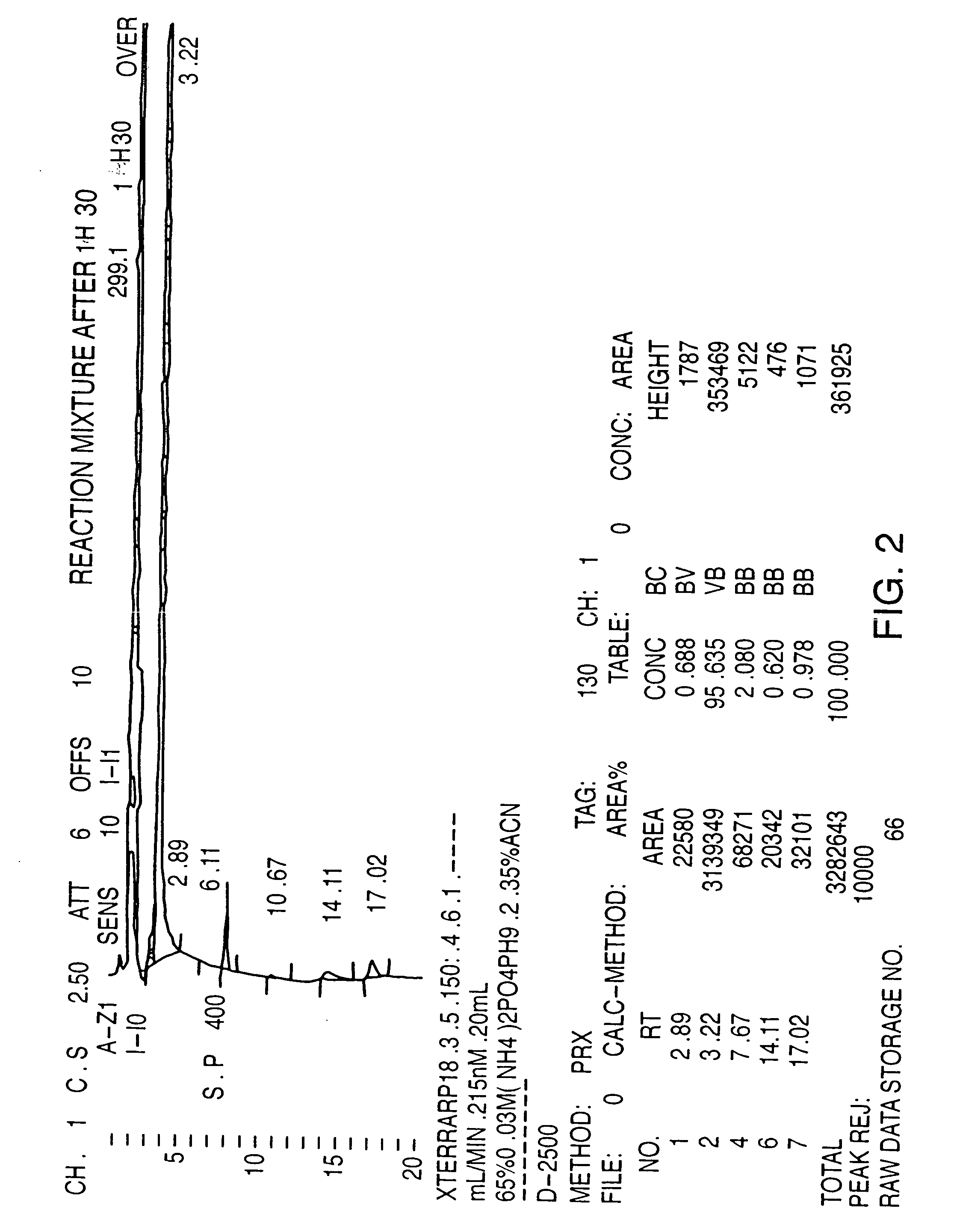
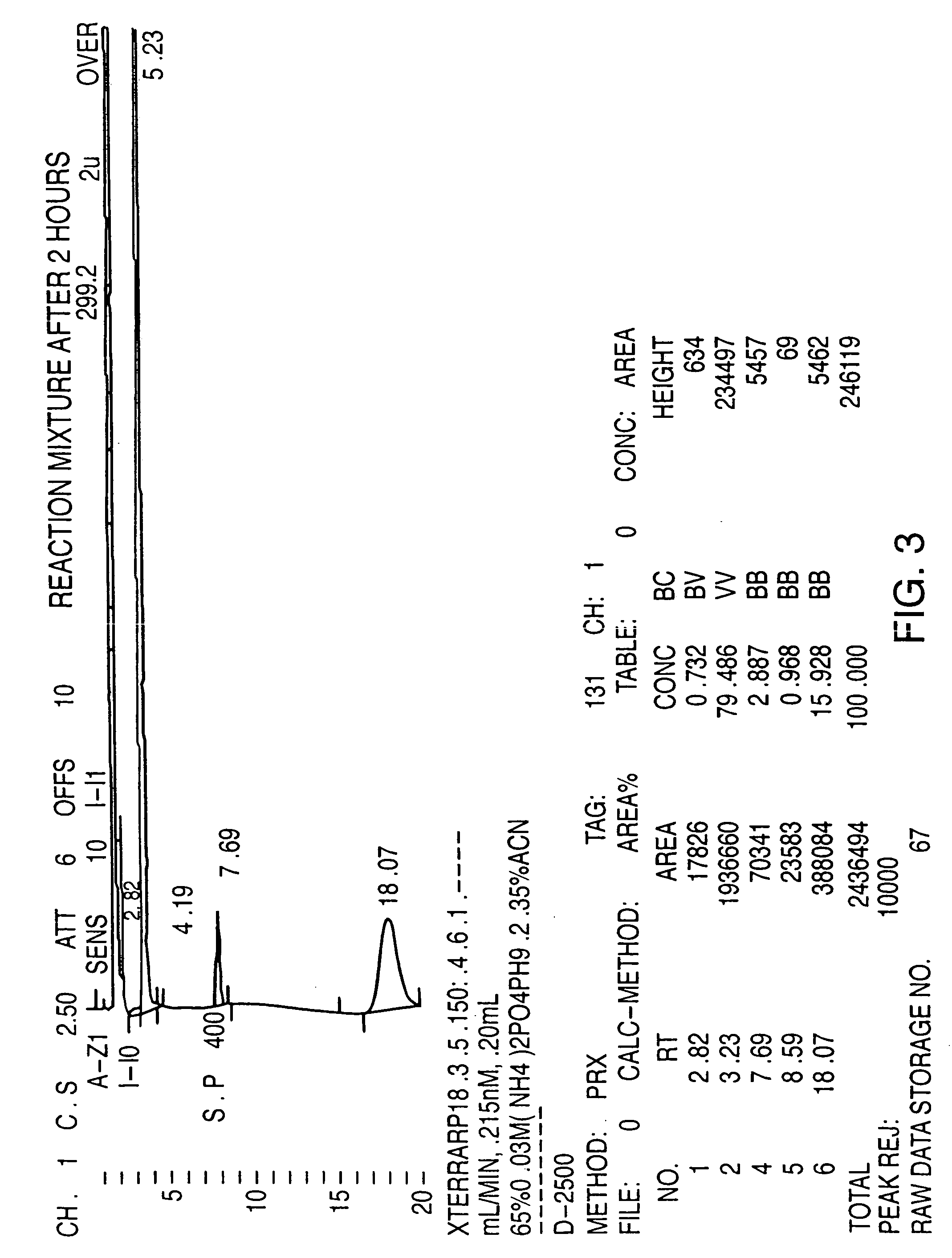
[0059] PMA (30 grams—containing approximately 2.6% by HPLC area percentage of the corresponding alkoxy impurity) was added to a solution of HBr (180 ml, 48%). The reaction mixture was then heated to strong reflux for about 1.50 hours until completion of the reaction. The reaction was followed by HPLC until the area percentage of the peak representing the alkoxy impurity is less than 0.5%. The reaction mixture was then allowed to cool to OEC, and was then basified to pH=11.5 with a solution 40% NaOH to increase the solubility of the corresponding phenol in the aqueous phase. Methylene chloride (150 ml) was then added, and the aqueous phase was extracted 3 times with methylene chloride (3×150 ml). The organic phase was then dried on sodium sulfate, filtrated and evaporated under reduced pressure, leaving about 15 g of methylene chloride. The oily mixture was then seeded with PMA crystal and after 1 hour the e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| HPLC | aaaaa | aaaaa |
| polarity | aaaaa | aaaaa |
| organic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com