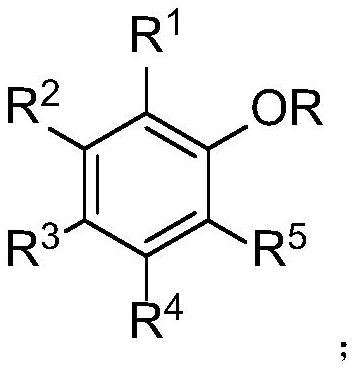
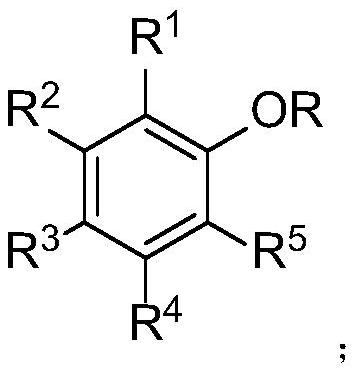
Selective ether bond breaking method of aryl alkyl ether
An aryl alkyl ether and selective technology, which is applied in the field of ether bond cleavage of aryl alkyl ethers, can solve problems such as insufficient demethylation chemical selectivity, and achieves low price, wide application range and chemical selectivity. improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
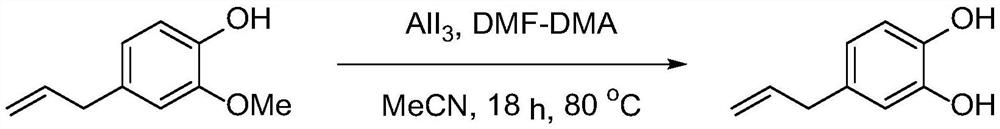
[0024] Embodiment 1 (eugenol demethylation)
[0025]
[0026] In a 100ml round bottom flask, add acetonitrile (40ml), aluminum triiodide (2.242g, 5.5mmol) and N,N-dimethylformamide dimethyl acetal (0.894g, 7.5mmol), at 80 ℃ Stir for 15min, add eugenol (0.821g, 5mmol), continue to stir at 80°C for 18h, quench with 2M dilute hydrochloric acid (10ml) after the reaction is complete, then extract three times with ethyl acetate (50ml), combine the organic phases, first Wash with a saturated aqueous solution of sodium thiosulfate (10ml), then dry with anhydrous magnesium sulfate, filter, and the filtrate is desolvated with a rotary evaporator, and the residue is subjected to flash column chromatography (eluent is petroleum ether / ethyl acetate=4 : 1, volume ratio) purification to obtain 0.615g 4-allyl catechol (white solid, yield 82%).
[0027] R f =0.34 (petroleum ether / ethyl acetate=3:1), melting point 44-45°C.
[0028] 1 H NMR (400MHz, CDCl 3 )δ6.76(d, J=8.1Hz, 1H), 6.68(d,...
Embodiment 2
[0035] Embodiment 2 (eugenol demethylation)
[0036]
[0037] Add acetonitrile (40ml), aluminum triiodide (2.242g, 5.5mmol) and dimethyl carbonate (0.901g, 10mmol) into a 100ml round bottom flask, stir at 80°C for 15min, add eugenol (0.821g, 5mmol), continue to stir at 80°C for 18h, quench with 2M dilute hydrochloric acid (10ml) after the reaction is complete, then extract three times with ethyl acetate (50ml), combine the organic phases, and first use a saturated aqueous solution of sodium thiosulfate (10ml) Washing, then drying with anhydrous magnesium sulfate, filtering, the filtrate was removed with a rotary evaporator, and the residue was purified by flash column chromatography (eluent: petroleum ether / ethyl acetate=4:1, volume ratio) to obtain 0.667 g 4-allyl catechol (white solid, yield 88%).
Embodiment 3
[0038] Embodiment 3 (eugenol demethylation)
[0039]
[0040]Acetonitrile (40ml), aluminum triiodide (2.242g, 5.5mmol) and N-methylformamide dimethyl acetal (0.788g, 7.5mmol) were added to a 100ml round bottom flask, stirred at 80°C for 15min, Add eugenol (0.821g, 5mmol), continue stirring at 80°C for 18h, quench with 2M dilute hydrochloric acid (10ml) after completion of the reaction, then extract three times with ethyl acetate (50ml), combine the organic phases, and Wash with a saturated aqueous solution of sodium sulfate (10ml), dry over anhydrous magnesium sulfate, filter, remove the solvent from the filtrate with a rotary evaporator, and pass the residue through flash column chromatography (the eluent is petroleum ether / ethyl acetate=4:1, volume ratio) to obtain 0.595 g of 4-allyl catechol (white solid, yield 79%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com