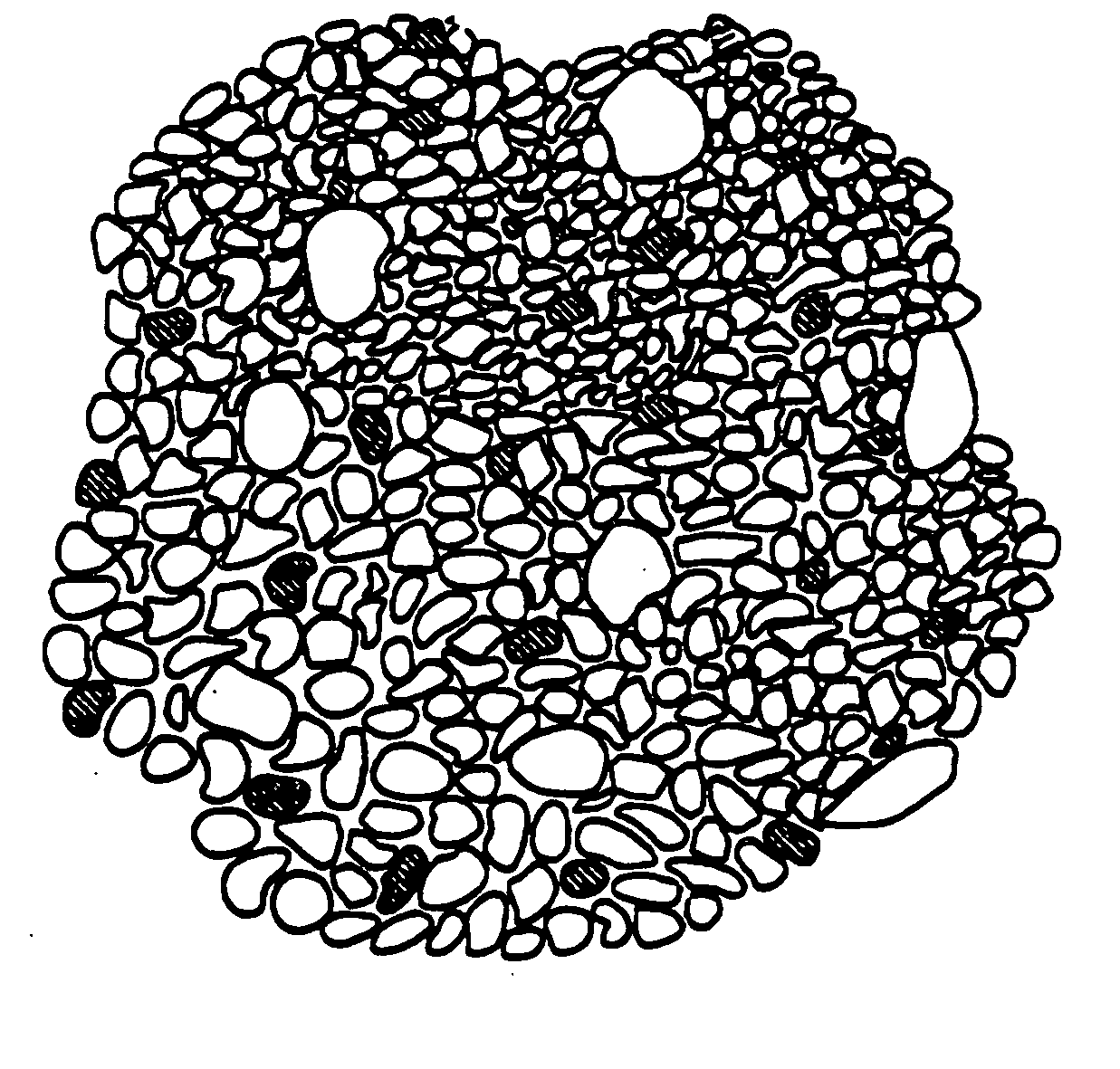
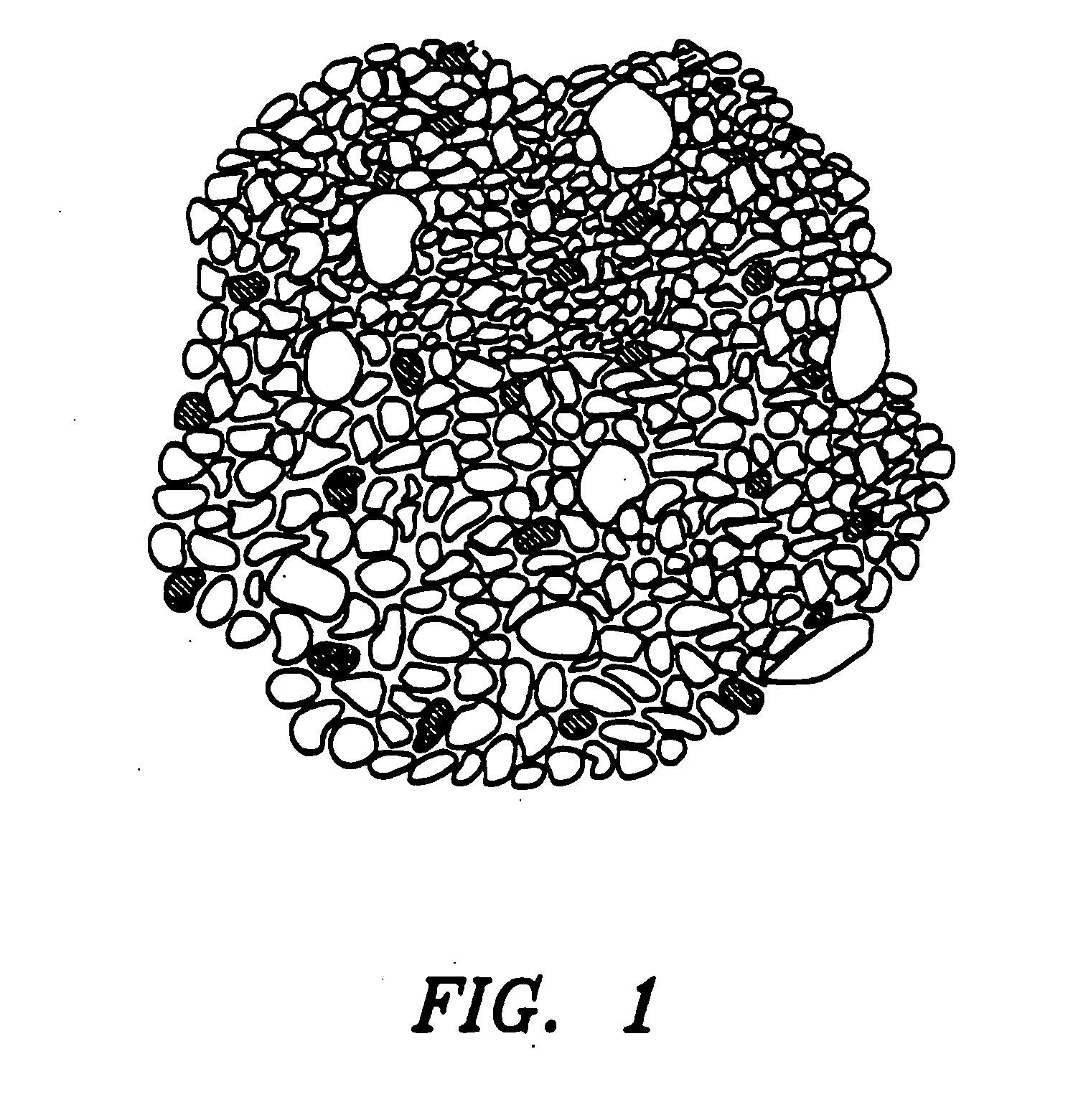
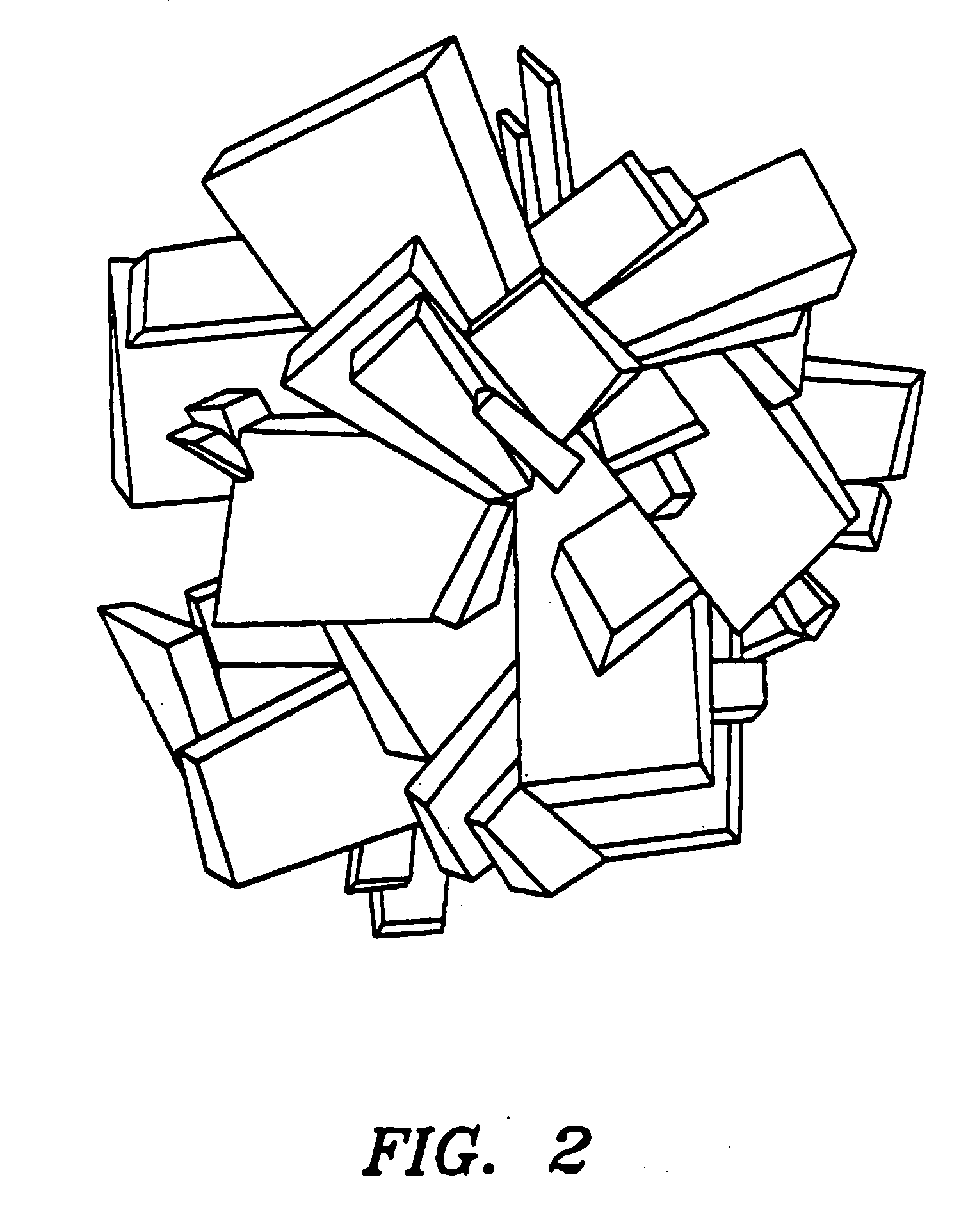
Inorganic shaped bodies and methods for their production and use
a technology of inorganic shaped bodies and fabrication methods, which is applied in the field of preparation of porous inorganic shaped bodies, can solve the problems of limited stoichiometric control, crystal morphology, surface properties, and ultimately reactivity in the body, and achieve the effects of improving uniformity, biological activity, and catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Low Temperature Calcium Phosphate Powders
[0143] An aqueous solution of 8.51 g 50 wt % hypophosphorous acid, H3PO2 (Alfa / Aesar reagent #14142, CAS #6303-21-5), equivalent to 71.95 wt % [PO4]−3 was combined with 8.00 g distilled water to form a clear, colorless solution contained in a 250 ml Pyrex beaker. To this solution was added 22.85 g calcium nitrate tetrahydrate salt, Ca(NO3)2.4H2O (ACS reagent, Aldrich Chemical Co., Inc. #23,712-4, CAS #13477-34-4), equivalent to 16.97 wt % Ca. The molar ratio of Ca / phosphate in this mixture was 3 / 2 and the equivalent solids level [as Ca3(PO4)2] was 25.4 wt %. Endothermic dissolution of the calcium nitrate tetrahydrate proceeded under ambient temperature conditions, eventually forming a homogeneous solution. Warming of this solution above 25° C. initiated a reaction in which the solution vigorously bubbled while evolving red-brown acrid fumes characteristic of NOx (g). The sample turned into a white, pasty mass which foamed and pulsed with per...
example 2
Low Temperature Calcium Phosphate Powder
[0148] Example 1 was repeated using five times the indicated weights of reagents. The reactants were contained in a 5½″ diameter Pyrex crystallizing dish on a hotplate with no agitation. Warming of the homogeneous reactant solution above 25° C. initiated an exothermic reaction which evolved red-brown acrid fumes characteristic of NOx (g). Within a few seconds following onset of the reaction, the sample turned into a white, pasty mass which continued to expel NOx (g) for several minutes. After approximately five minutes, the reaction was essentially complete leaving a damp solid mass which was hot to the touch. This solid was cooled to room temperature under ambient conditions for approximately 20 minutes and divided into two portions prior to heat treatment.
[0149] Heat treatment and X-ray diffraction of this solid were conducted as described in Example 1. Following heat treatment in air, XRD indicated the fired solids to be composed of:
Hea...
example 3
Low Temperature Calcium Phosphate Powders
[0150] An aqueous solution of 8.51 g 50 wt % H3PO2 was combined with 8.00 g of 25.0 wt % aqueous solution of calcium acetate monohydrate, Ca(O2CCH3)2.H2O (ACS reagent, Aldrich Chemical Co., Inc. #40,285-0, CAS 5743-26-0), equivalent to 5.69 wt % Ca, to give a clear, colorless solution contained in a 250 ml Pyrex beaker. To this solution was added 20.17 g Ca(NO3)2.4H2O salt. The molar ratio of Ca / phosphate in this mixture was 3 / 2 and the equivalent solids level [as Ca3(PO4)2] was 27.3 wt %. Endothermic dissolution of the calcium nitrate tetrahydrate salt proceeded giving a homogeneous solution once the sample warmed to room temperature. Further warming of this solution to >25° C. on a hotplate initiated a reaction which proceeded as described in Example 1. After approximately three minutes, the reaction was essentially complete leaving a moist, white, crumbly solid which was hot to the touch and which smelled of acetic acid. After cooling to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallite size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pore diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com