IGF antagonist peptides
a technology of peptides and antagonists, applied in the field of peptides, can solve the problems of limited use of igf and igfbp in screening, preventing, or treating disease, and restricting the generation of specific igf-1 antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Procedure
Construction of Polyvalent Naïve Peptide Libraries
[0208] Libraries were constructed using the method of Sidhu et al., Methods Enzymol., 328: 333-363 (2000) with a phagemid containing an IPTG-inducible Ptac promoter driving the expression of open reading frames encoding fusion proteins of the following form: the STII secretion signal (MKKNIAFLLASMFVFSIATNAYA; SEQ ID NO:8), followed by a random peptide (i.e., a member of the naïve peptide library), followed by a linker (GGGSGGG; SEQ ID NO:9), followed by the M13 gene-8 major coat protein
(AEGDDPAKAAFNSLQASATEYIGYAWAMVVVIVGATIGIKLFKKFTSKAS; SEQ ID NO:10).
Twenty-two different peptide libraries were constructed as shown in Table 1. Phage displaying the naïve libraries were purified by precipitation with PEG / NaCl as described in Sidhu et al., supra, and stored frozen at −70° C.
Isolation of IGF-1 Binding Peptides from Naïve Peptide-Phage Libraries
[0209] IGF-1 was obtained in house as described in U.S. Pat. No...
example 2
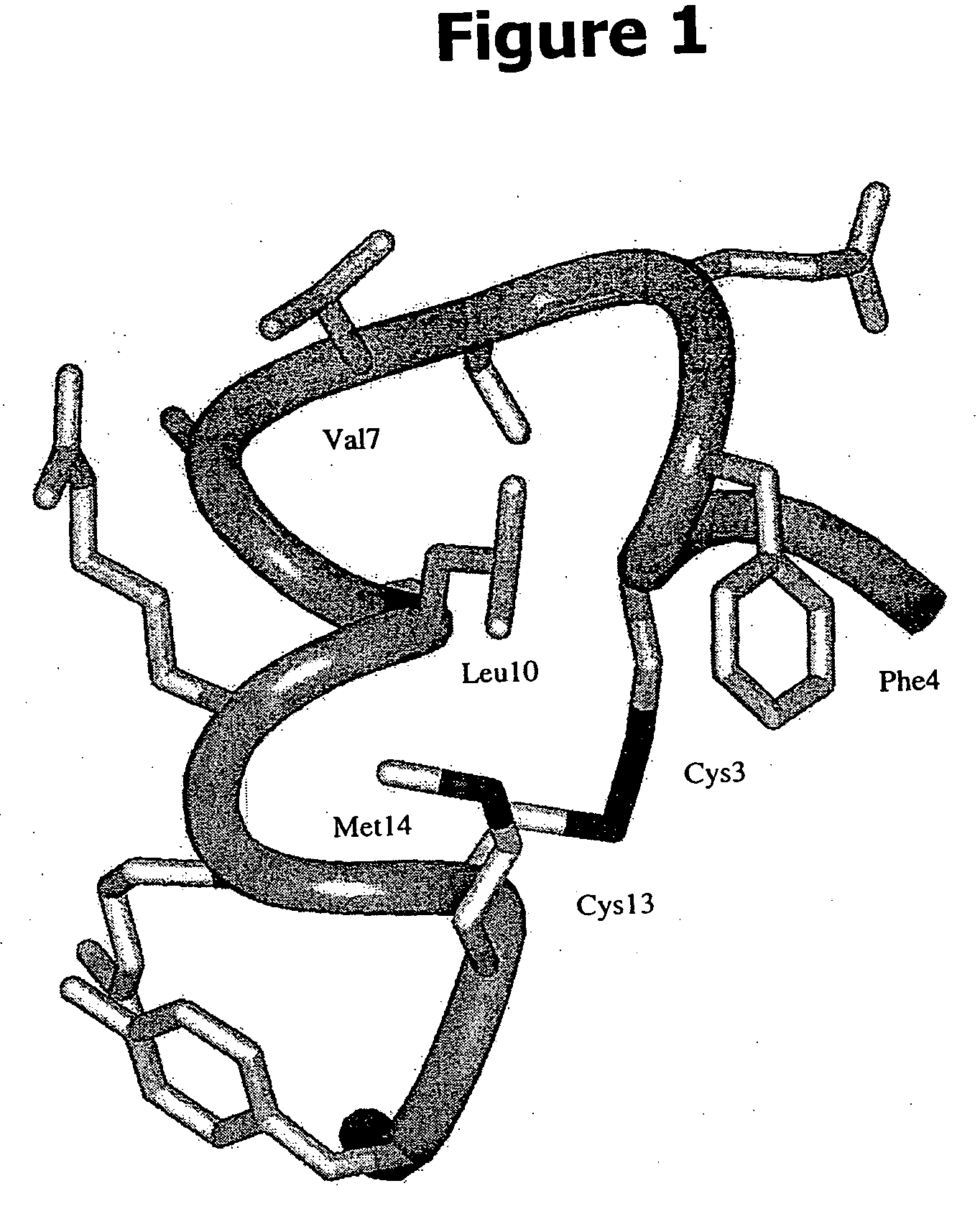
Structure Determination of IGF-F1-1 by NMR
[0233]1H NMR data were collected on peptide IGF-F1-1 either in pure H2O solution (30° C., pH 5.1 and 5.0 millimolar concentration) or in H2O containing 6% (v / v) d6-DMSO (40° C., pH 5.2 and at a concentration of 6.7 millimolar). In addition to one-dimensional spectra, two-dimensional double-quantum-filtered correlation spectroscopy (2QF-COSY), total correlation spectra (TOCSY), and rotating-frame Overhauser effect spectra (ROESY) were collected. The experiments were recorded as described by Cavanagh et al. in Protein NMR Spectroscopy Principles and Practice (Academic Press, San Diego; ISBN 0-12-164490-1, 1995), except that pulsed-field gradients were used for coherence selection in the 2QF-COSY (van Zijl et al., J. Magn. Reson., 113A: 265-270 (1995)) and excitation sculpting was used to suppress the water resonance in the TOCSY and ROESY experiments (Hwang and Shaka, J. Magn. Reson., 112A: 275-279 (1995)). After lyophilization and dissoluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| mass median dynamic diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com