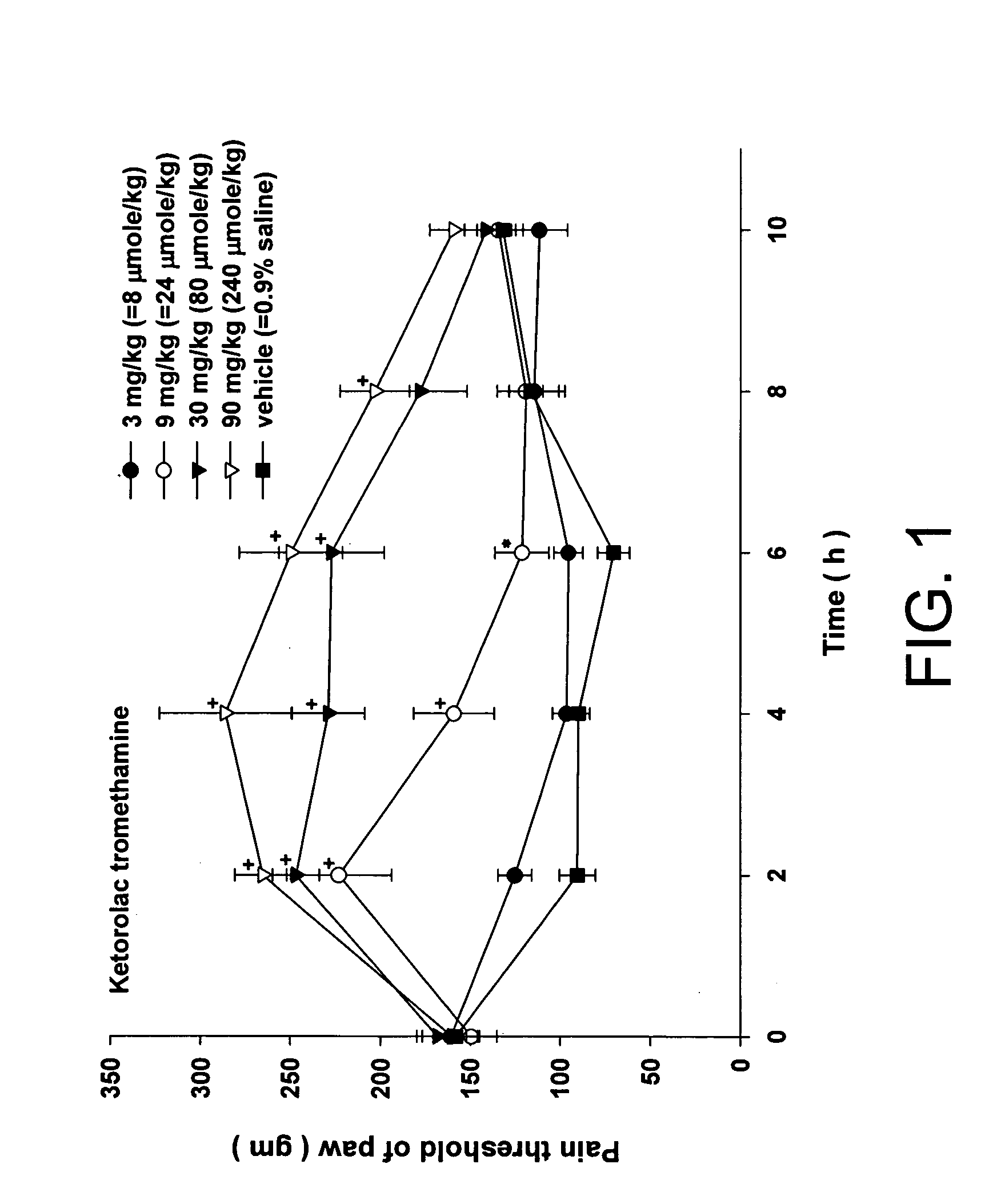
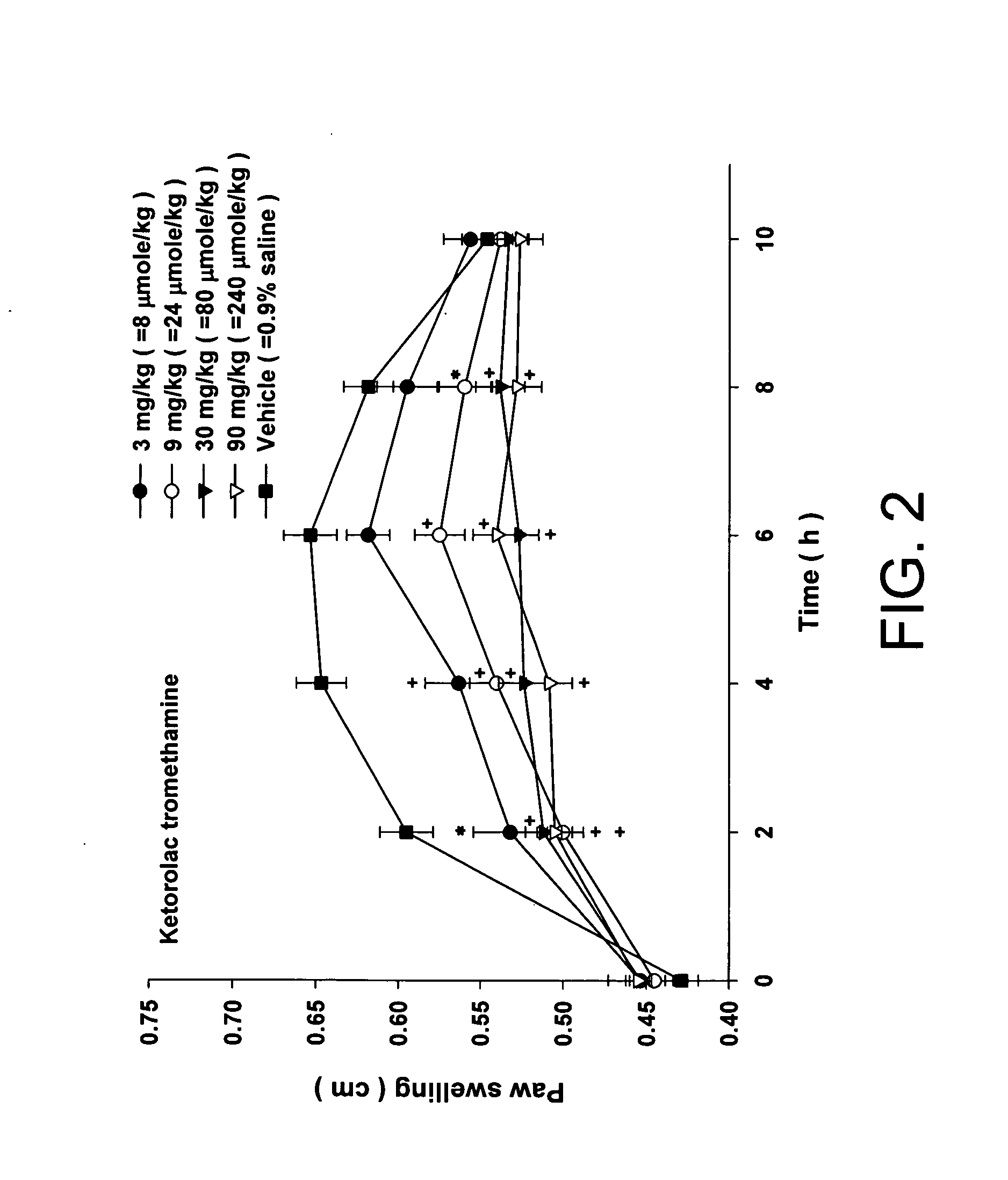
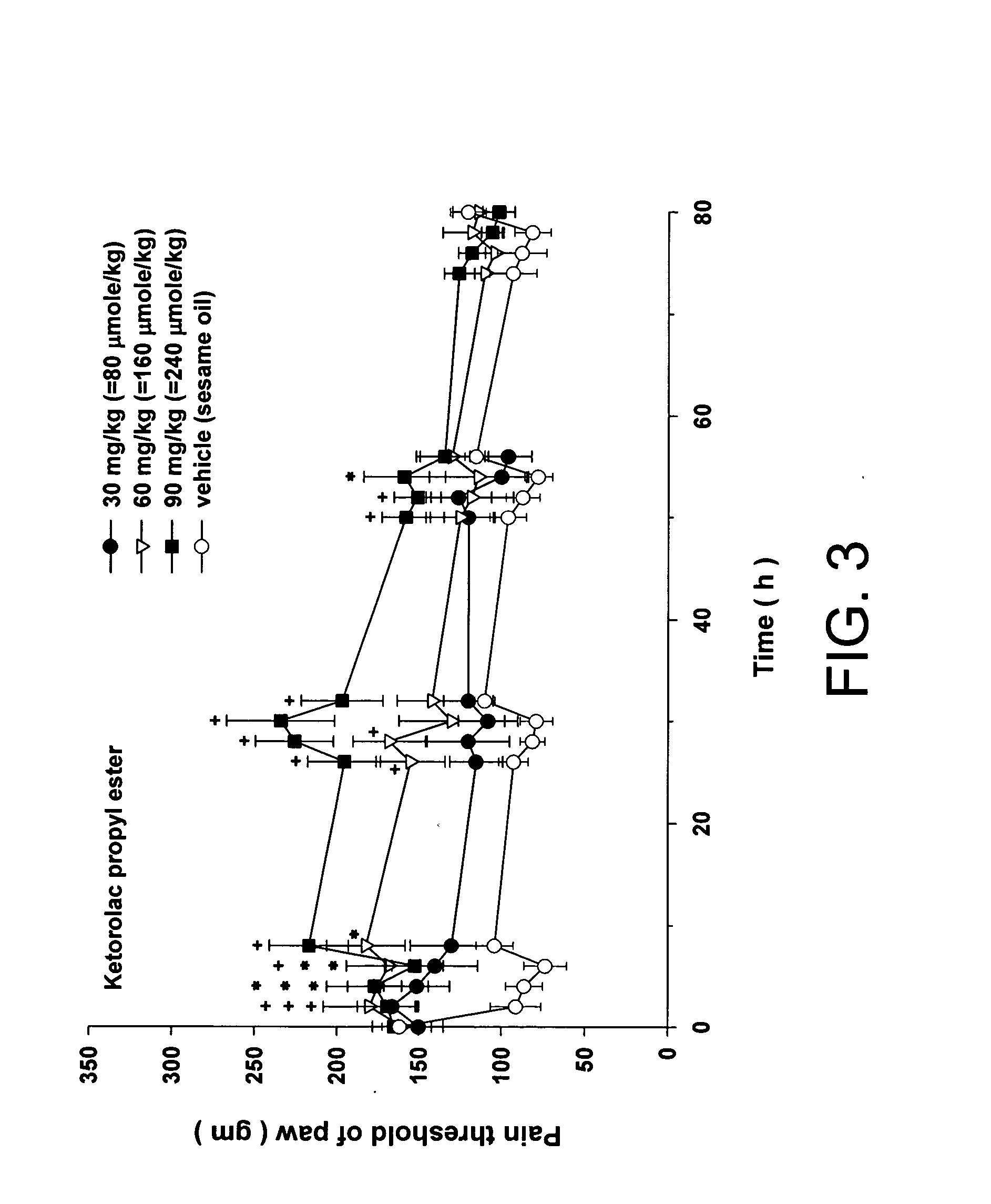
Injectable long-acting analgesic composition comprising an ester derivative of ketorolac
an analgesic composition and ester derivative technology, applied in the direction of drug compositions, heterocyclic compound active ingredients, biocide, etc., can solve the problems of severe acute pain, no known ketorolac esters applied, and the short-term management of severe pain and acute pain of ketorolac tromethamine, etc., to achieve the effect of prolonging the anti-inflammatory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0074] Table 1 shows the chemical structures of the preferred ketorolac ester derivatives obtained in the following Synthesis Examples.
TABLE 1The chemical structures of ketorolac, ketorolac tromethamine, andketorolac ester derivatives obtained in the Synthesis Examples.Compound nameChemical structureKetorolacKeto—HKetorolacKeto.TromethaminetromethamineKetorolac propyl esterKetorolac pentyl esterKetorolac tert-butyl esterKetorolac hexyl esterKetorolac heptyl esterKetorolac decyl esterKetorolac cetyl esterKetorolac benzyl ester
[0075] The ketorolac ester derivatives listed in Table 1 can be synthesized by suitable known methods other than those described below.
Synthesis Ex. 1
Preparation of Ketorolac Propyl Ester
[0076] Ketorolac tromethamine was purchased from Sigma (Saint Louis, Mo., USA). Ketorolac was obtained from its tromethamine salt using a precipitation method. Following adding 1 N HCl drop by drop into a ketorolac tromethamine solution, ketorolac was precipitated. The coll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com