Use of lipid conjugates in the treatment of infection
a technology of conjugates and lipids, applied in the field of infection treatment with lipid conjugates, can solve the problems of difficult to distinguish viral replicative mechanisms from host replicative processes, relatively few effective pharmaceutical compositions intended or adapted for antiviral, antifungal, etc., and achieve the effect of suppressing, inhibiting, or treating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
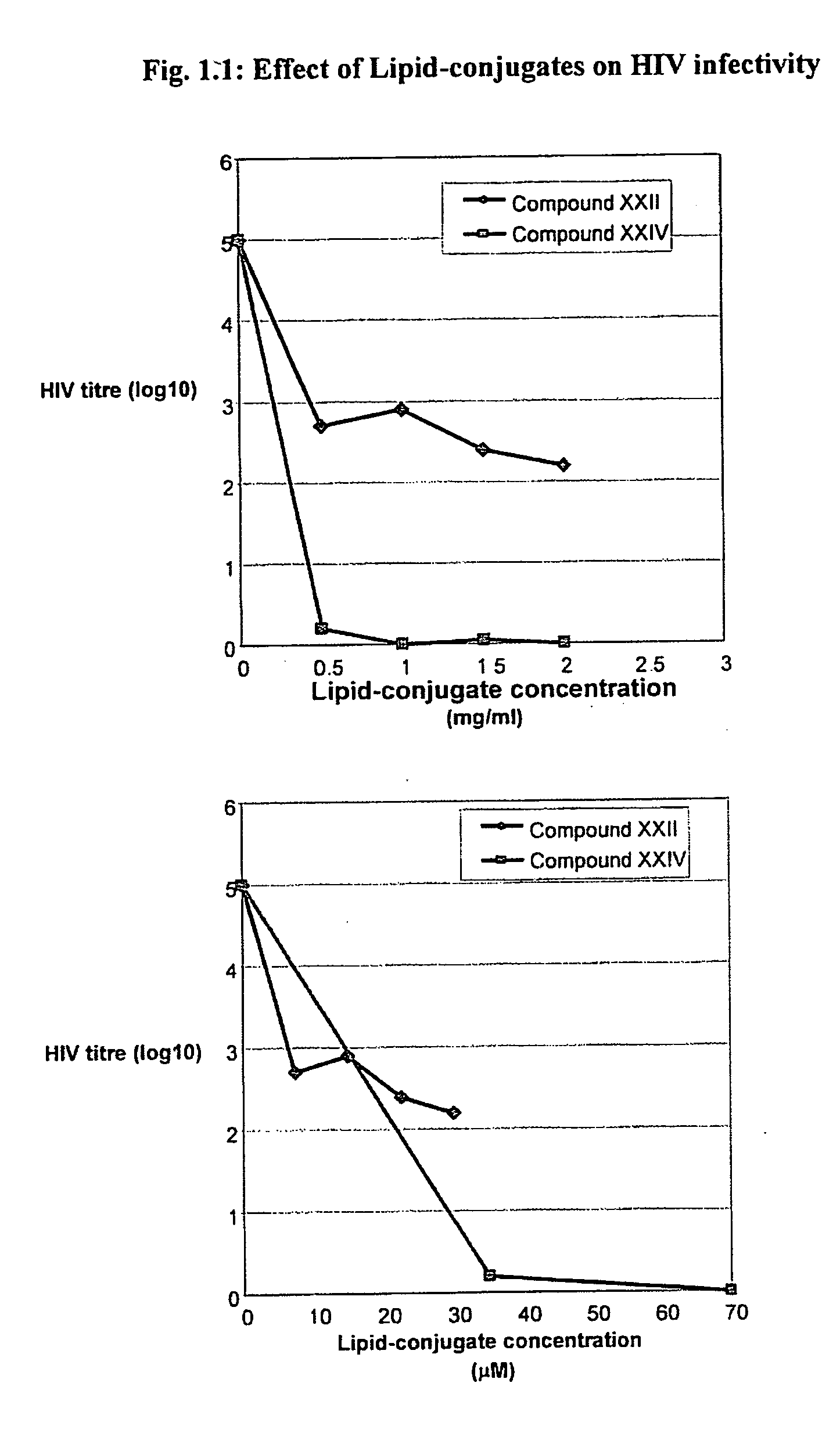
[0453] The Lipid-conjugates are effective in the prophylaxis and treatment of viral infection, particularly infections due to the human immunodeficiency virus (HIV), human influenza virus and vaccinia virus. This is demonstrated for HIV in Experiments 1.1-1.3 below, for human influenza virus in Experiment 1.4-1.5, and for vaccinia in Experiment 1.6 below.
[0454] Viral infection is the cause of a number of human and animal diseases throughout the world. The process of viral infection comprises several stages, including attachment, penetration, uncoating, replication, maturation, release and reinfection. In order to assess the ability of Lipid-conjugates to prevent viral infection, human cell lines were incubated with a preparation of a viral agent, and the ability of the virus to infect cells is compared in the presence and absence of Lipid-conjugate.
[0455] Experiment 1.1: To demonstrate that the Lipid-conjugates are capable of preventing HIV infection of target cell...
example 2
Treatment of Chlamydia Infection
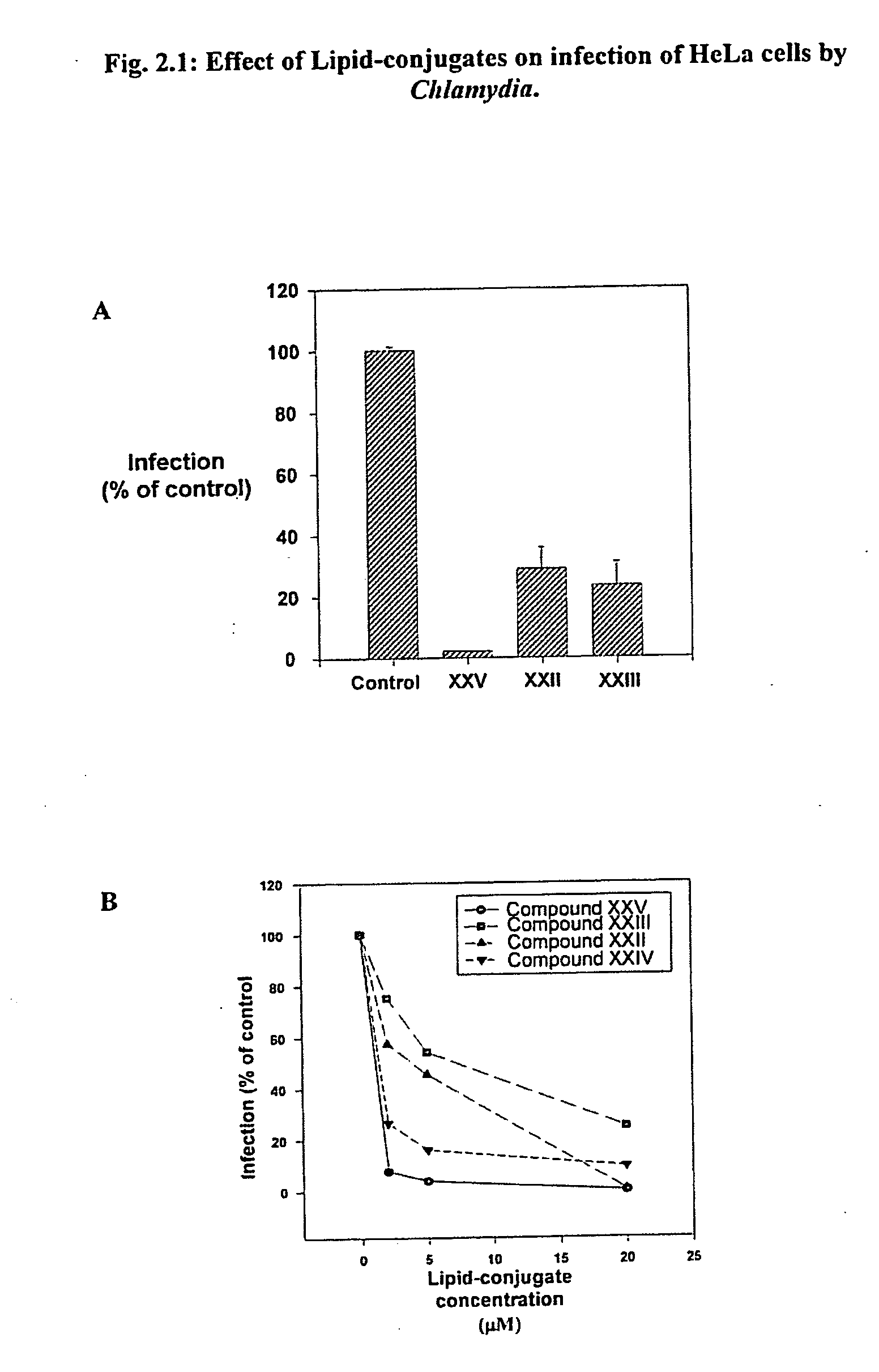
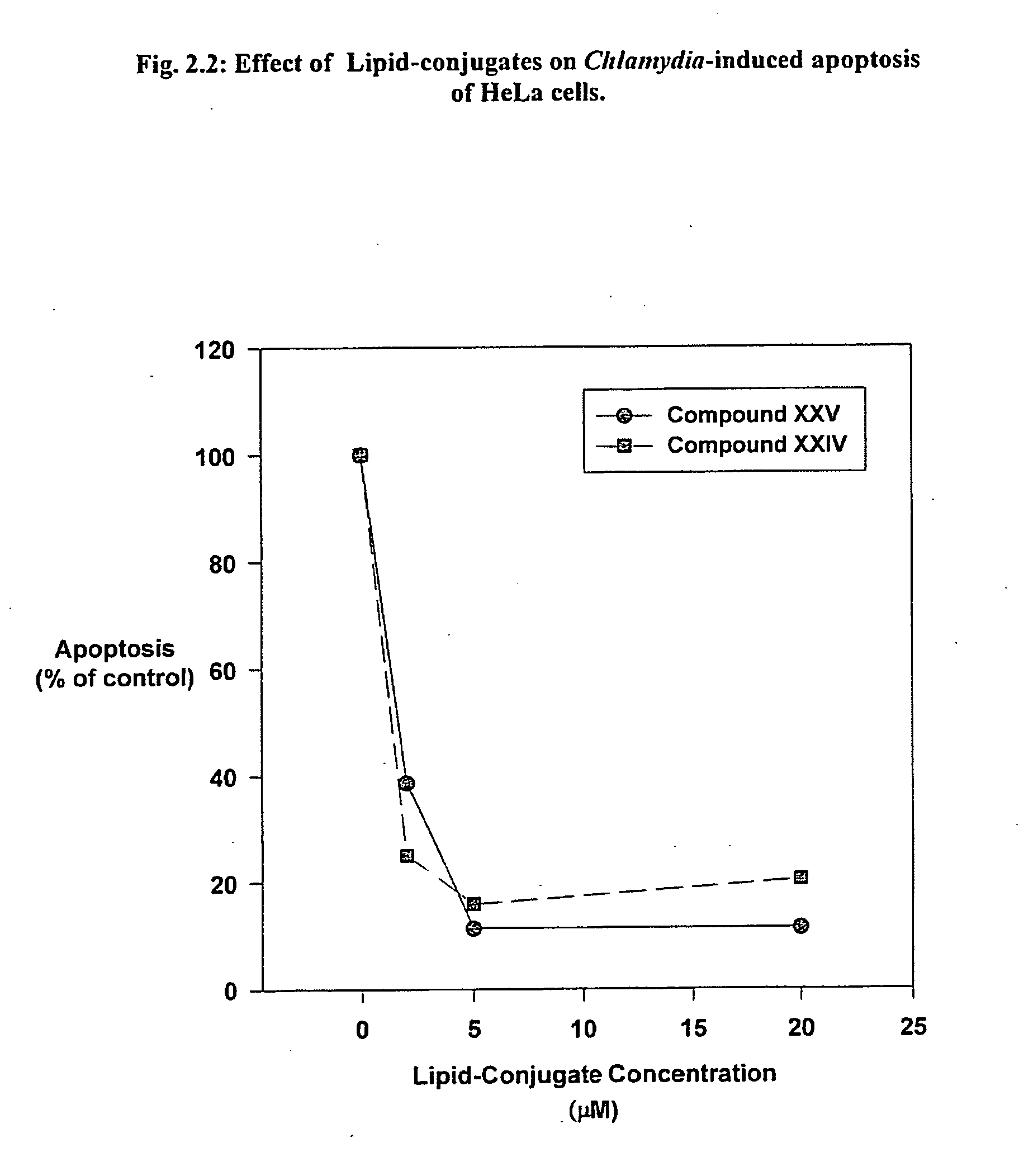
[0491] Intracellular bacterial parasites are one of the most prevalent forms of sexually transmitted disease and are frequently intractable to conventional antibiotic therapy. Infection of the female genital tract with chlamydia species is a salient example. Experiment 2.1 demonstrates the ability of Lipid-conjugate treatment to prevent infection of HeLa cells by Chlamydia. Human cervical adenocarcinoma cell line, HeLa 229 (ATCC, Manassas, Calif.), were cultured and incubated with the phospholipid conjugates (20 micromolar) for 30 min, then incubated with Chlamydia psittaci (an avian form of Chlamydia trachomatis) (guinea pig inclusion conjunctivitis serologically variant strains (serovars)) for 24 hr. Infected cells were detected by cytofluorometry (FACS) using FITC-conjugated anti-Chlamydia antibody (FIG. 2.1A).
[0492]FIG. 2.1B depicts the dose response of the Lipid-conjugates' inhibitory effect on infection of HeLa cells by Chlamydia. HeLa cells w...
example 3
Obstructive Respiratory Disease
[0495] In asthma, the impeded airflow is due to airway obstruction which is the result of constriction and obstruction of luminal vessels of the lungs. In order to determine the effect of Lipid-conjugates on obstructive respiratory disease, contraction of smooth muscle preparations isolated from airways was induced in the presence and absence of Lipid-conjugates. This is a widely-accepted experimental system to investigate airway constriction.
[0496] A muscle preparation (tracheal rings) was isolated from rats (Experiments 3.1-3.3) and from guinea pigs (Experiments 3.4-3.5). Muscle contraction was measured by attachment of the muscle to a pressure transducer, which works much like a spring. Administration of asthmatogenic substances such as endothelin-1 (ET) and acetylcholine (AcCh) induces muscle contraction. Endothelins are released upon vascular endothelial injury, and they activate macrophages and act as strong chemo-attractants for circulating mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com