Cardiac muscle function and manipulation
a technology of cardiac muscle and function, applied in the field of polypeptides, can solve the problems of reducing the mortality rate of heart failure only by, slowing the progress of end-stage heart failure progress, and unable to relieve symptoms in more than 60 years, so as to improve sarcoineric and cytoskeleton structure, improve cell-cell adhesion, and reduce the effect of dna synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
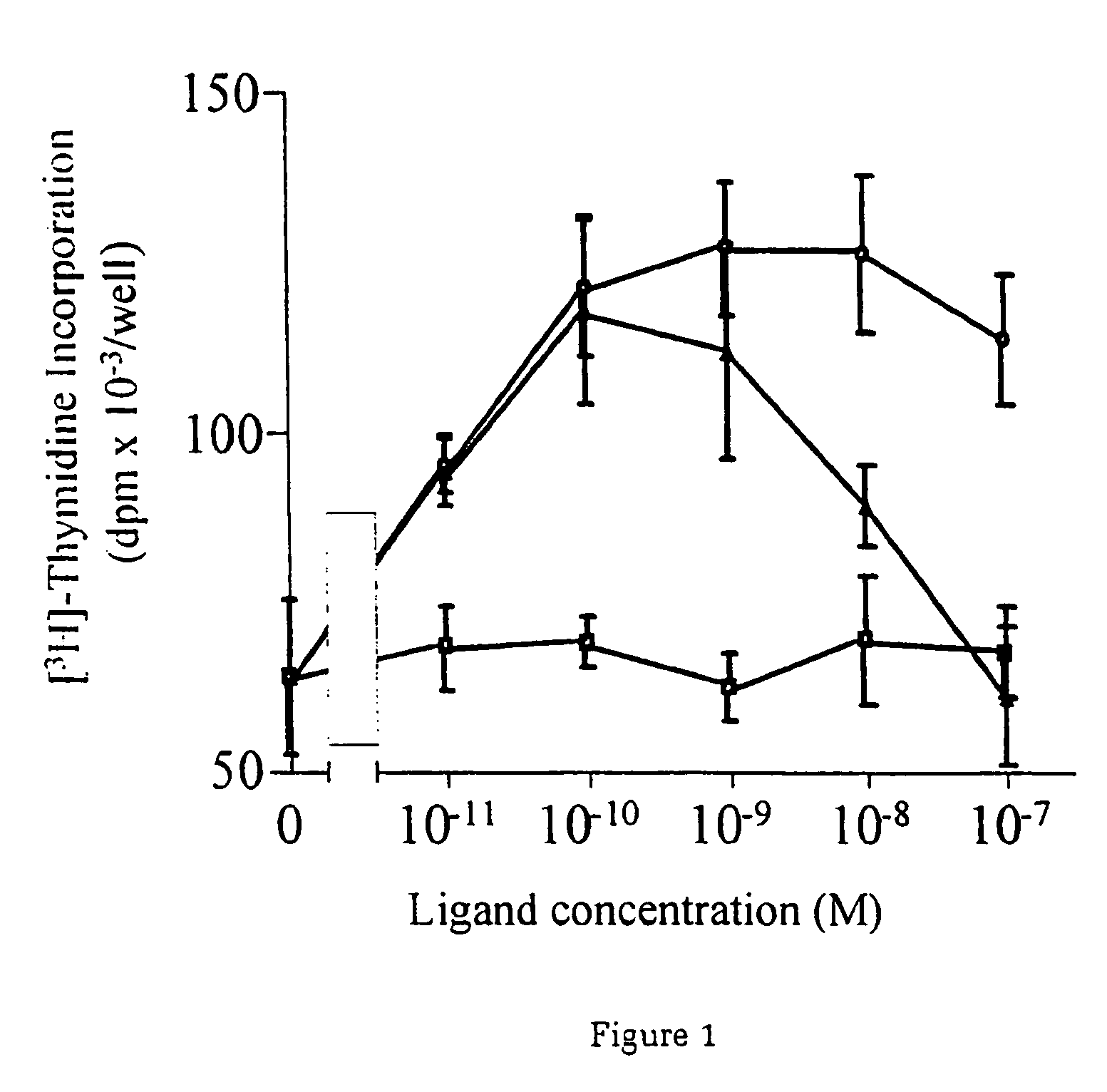
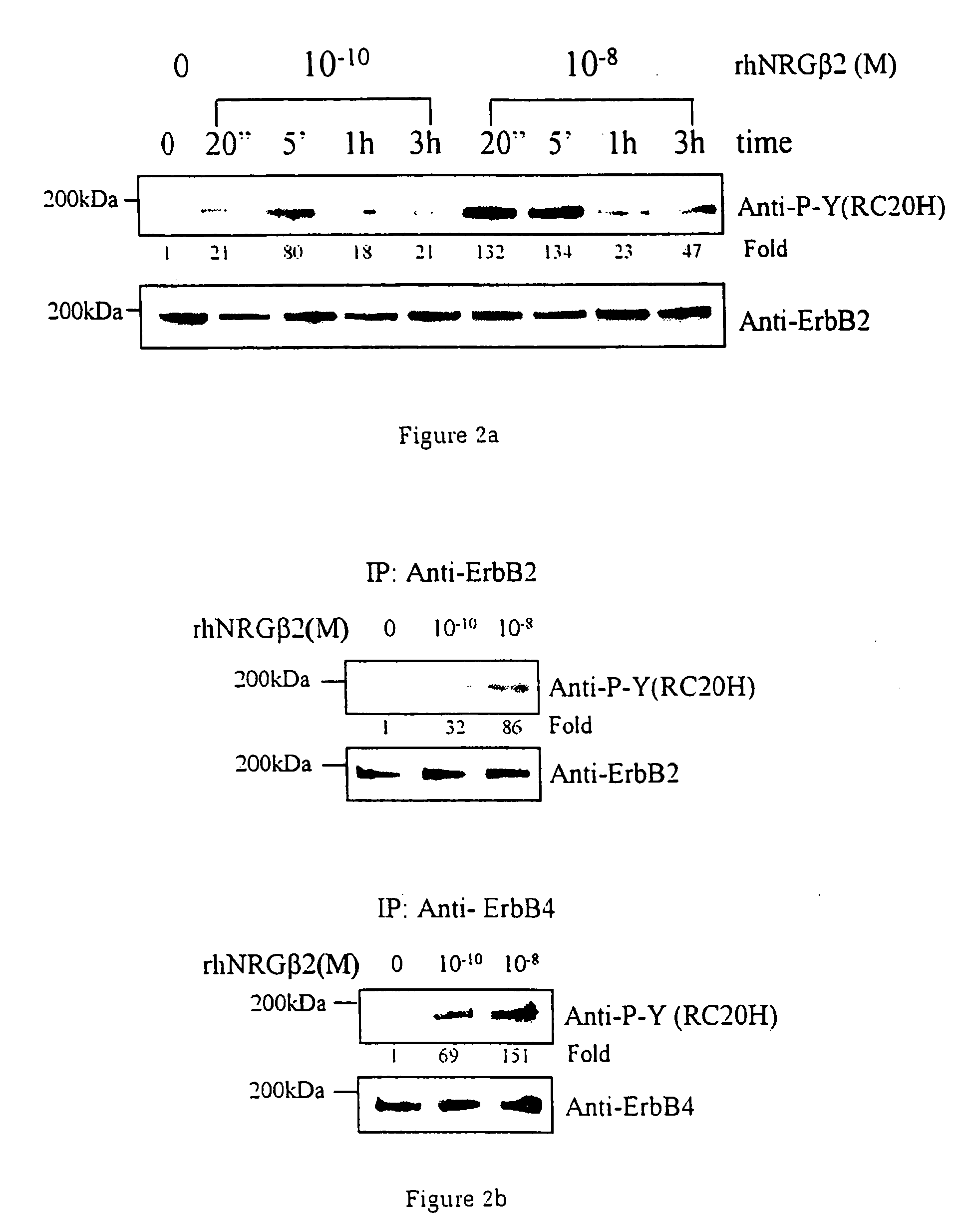
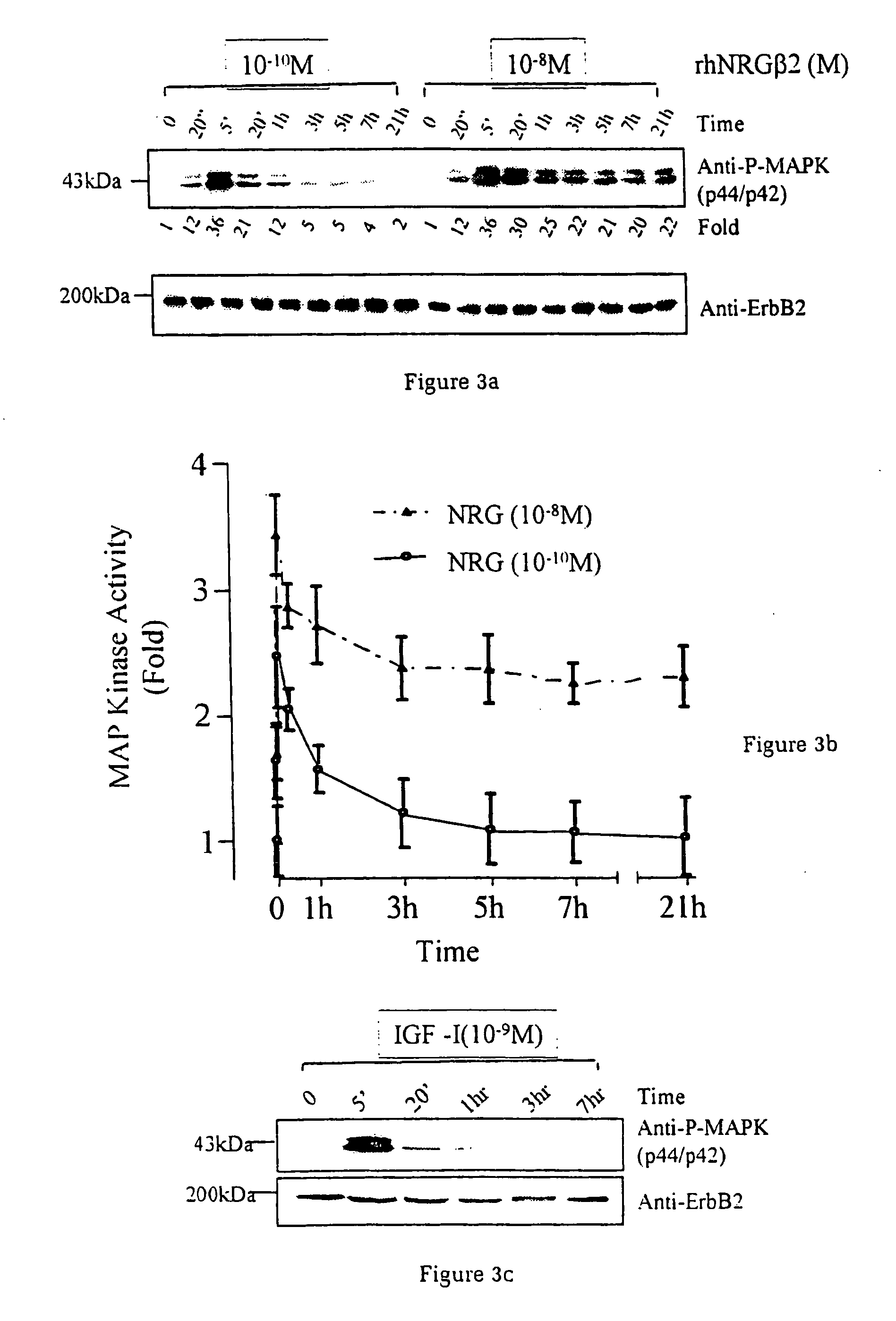
[0037] Utilising an ini vitro system of cardiac muscle cell differentiation, a role for neuregulin in stimulating the activation of the differentiation response in comparison with two well-defined hormonal and growth factor stimuli, α1-adrenergic agonists and IGF-1 has been demonstrated. The present inventor has demonstrated that neuregulin differentiation pathways exist within cardiac muscle cells, and that neuregulin polypeptides can activate these pathways. Since cardiac muscle cell differentiation includes the processes of organisation of sarcomeric structures and cell-cell adhesions, the invention, thus, provides a useful method for the treatment and prevention of cardiac muscle cell with disorganisation of the sarcomeric structures and cell-cell adhesions, and the enhancement of heart function in cardiomyopathy, and for identifying polypeptides or compounds which activate cardiac muscle differentiation pathways.
[0038] Before the methods of the invention are described, it is t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com