Adeno-associated virus serotype 1 nucleic acid sequences, vectors and host cells containing same
a technology of adeno-associated virus and nucleic acid sequence, which is applied in the field of recombinant viral vectors, can solve the problems that antibodies may significantly decrease the usefulness of aav vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Infectious Clone of AAV-1
[0076] The replicated form DNA of AAV-1 was extracted from 293 cells that were infected by AAV-1 and wild type adenovirus type 5.
[0077] A. Cell Culture and Virus
[0078] AAV-free 293 cells and 84-31 cells were provided by the human application laboratory of the University of Pennsylvania. These cells were cultured in Dulbecco's Modified Eagle Medium with 10% fetal bovine serum (Hyclone), penicillin (100 U / ml) and streptomycin at 37EC in a moisturized environment supplied with 5% CO2. The 84-31 cell line constitutively expresses adenovirus genes E1a, E1b, E4 / ORF6, and has been described previously [K. J. Fisher, J. Virol., 70:520-532 (1996)]. AAV-1 (ATCC VR-645) seed stock was purchased from American Type Culture Collection (ATCC, Manassas, Va.). AAV viruses were propagated in 293 cells with wild type Ad5 as a helper virus.
[0079] B. Recombinant AAV Generation
[0080] The recombinant AAV viruses were generated by transfection using an adenovirus...
example 2
Sequencing Analysis of AAV-1
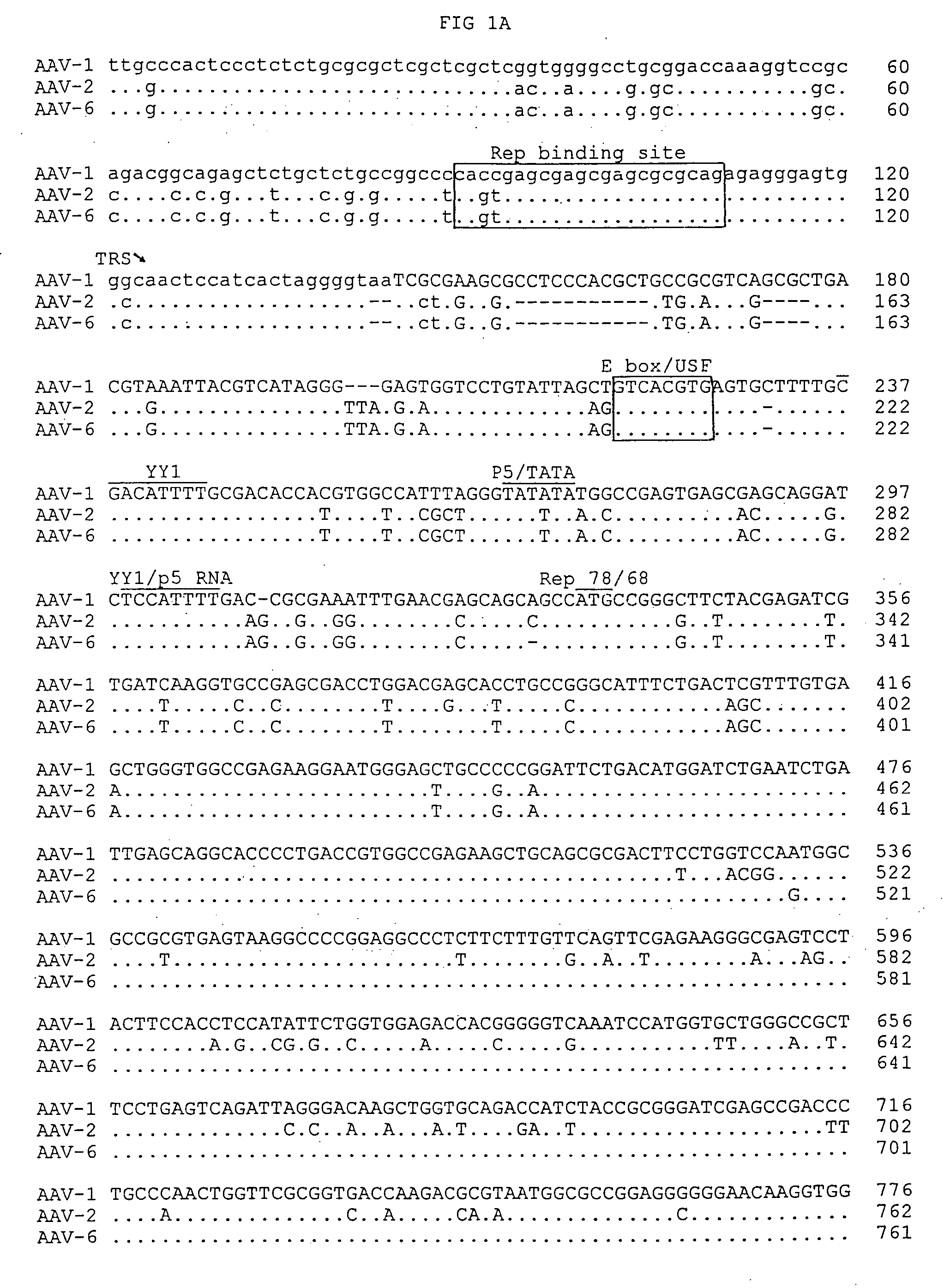
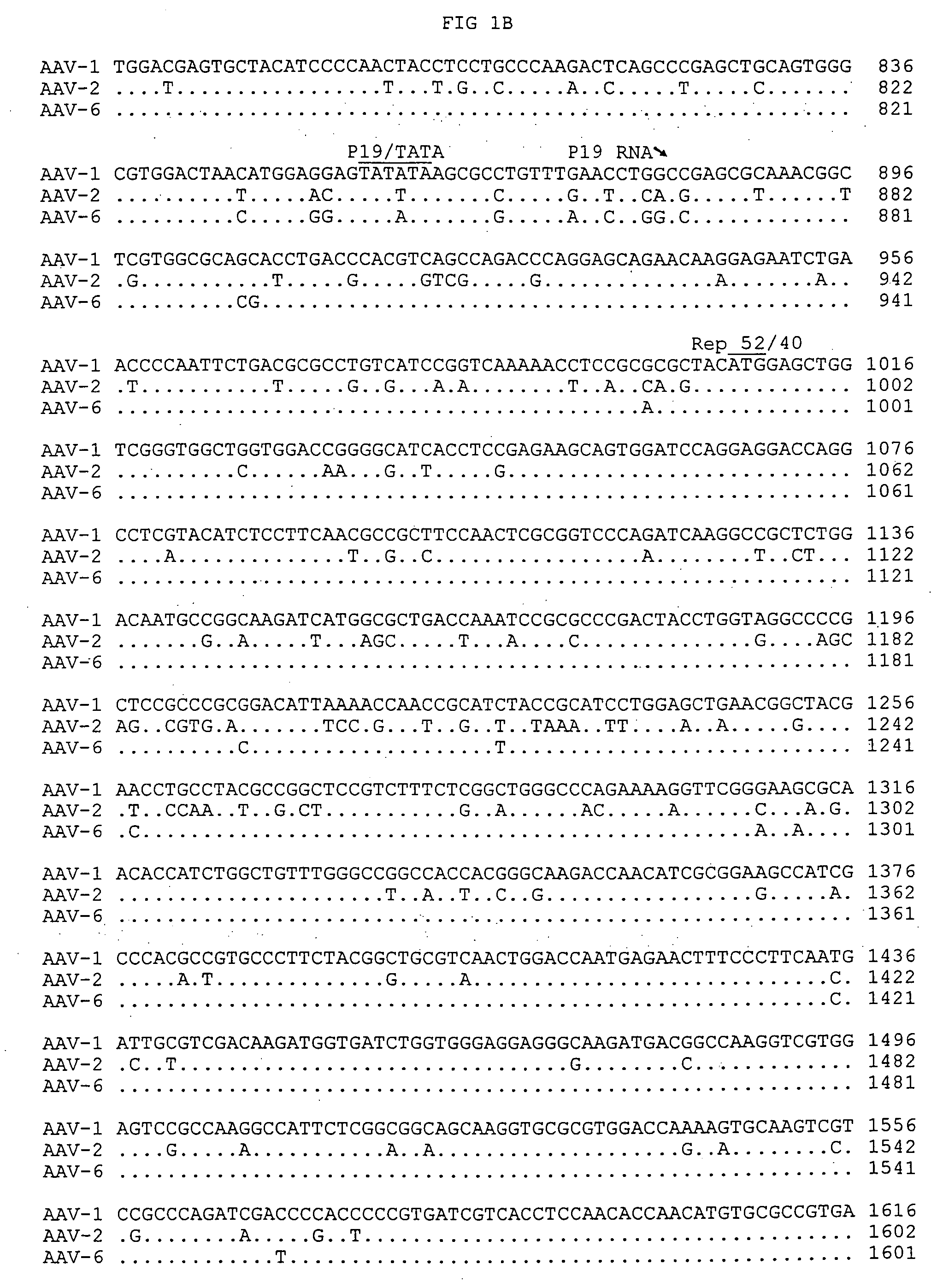
[0087] The entire AAV-1 genome was then determined by automatic sequencing and was found to be 4718 nucleotides in length (FIGS. 1A-1F). For sequencing, an ABI 373 automatic sequencer as used to determine the sequences for all plasmids and PCR fragments related to this study using the FS dye chemistry. All sequences were confirmed by sequencing both plus and minus strands. These sequences were also confirmed by sequencing two independent clones of pAV-BM, pAV-BL and pAV-BR. Since the replicated form of AAV-1 DNA served as the template for sequence determination, these sequences were also confirmed by sequencing a series of PCR products using original AAV-1 seed stock as a template.
[0088] The length of AAV-1 was found to be within the range of the other serotypes:
[0089] AAV-3 (4726 nucleotides), AAV-4 (4774 nucleotides), AAV-2 (4681 nucleotides), and AAV-6 (4683 nucleotides).
[0090] The AAV-1 genome exhibited similarities to other serotypes of adeno-ass...
example 3
Comparison of AAV-1 Sequences
[0095] The nucleotide sequences of AAV-1, obtained as described above, were compared with known AAV sequences, including AAV-2, AAV-4 and AAV-6 using DNA Star Megalign. This comparison revealed a stretch of 71 identical nucleotides shared by AAV-1, AAV-2 and AAV-6. See, FIGS. 1A-1F.
[0096] This comparison further suggested that AAV-6 is a hybrid formed by homologous recombination of AAV-1 and AAV-2. See, FIGS. 3A and 3B. These nucleotides divide the AAV-6 genome into two regions. The 5′ half of AAV-6 of 522 nucleotides is identical to that of AAV-2 except in 2 positions. The 3′ half of AAV-6 including the majority of the rep gene, complete cap gene and 3′ ITR is 98% identical to AAV-1.
[0097] Biologically, such recombination may enable AAV-1 to acquire the ability to transmit through the human population. It is also interesting to note that the ITRs of AAV-6 comprise one AAV-1 ITR and one AAV-2 ITR. The replication model of defective parvovirus can main...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| Nucleic Acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com