Aav vector compositions and methods for gene transfer to cells, organs and tissues
a technology of aav and composition, applied in the direction of drug composition, virus/bacteriophage, extracellular fluid disorder, etc., can solve the problems of need for repeated infusion, cost of treatment, risk of anti-therapeutic factor immune response,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0104]This example includes a description of various materials and methods.
[0105]Mice:
[0106]Male C57BL / 6J (WT) mice 8-10 weeks of age, n=5 per experimental group. The dog is a HB dog from the University of North Carolina Chapel Hill colony carrying a missense mutation in the FIX gene (Evans et al., Proc Natl Acad Sci USA 86:10095 (1989)).
[0107]AAV Vector Constructs:
[0108]The in vivo studies in mice were performed using a construct expressing human FIX under the control of the ApoE-hAAT liver specific promoter. The study in dogs used a nearly identical promoter and the canine FIX transgene.
[0109]Gene Transfer Methodology:
[0110]All vectors were delivered intravenously. In mice via the tail vein (a volume of 200 microliters per mouse was administered, vector was diluted in PBS). In dogs the vector was delivered via the saphenous vein.
[0111]FIX Expression Determination:
[0112]ELISA was used to measure FIX levels. In mice, the human FIX ELISA antibody pair (capture and secondary) is from ...
example 2
[0120]This example includes a description of human FIX gene transfer animal (Mice) studies and FIX expression after gene transfer.
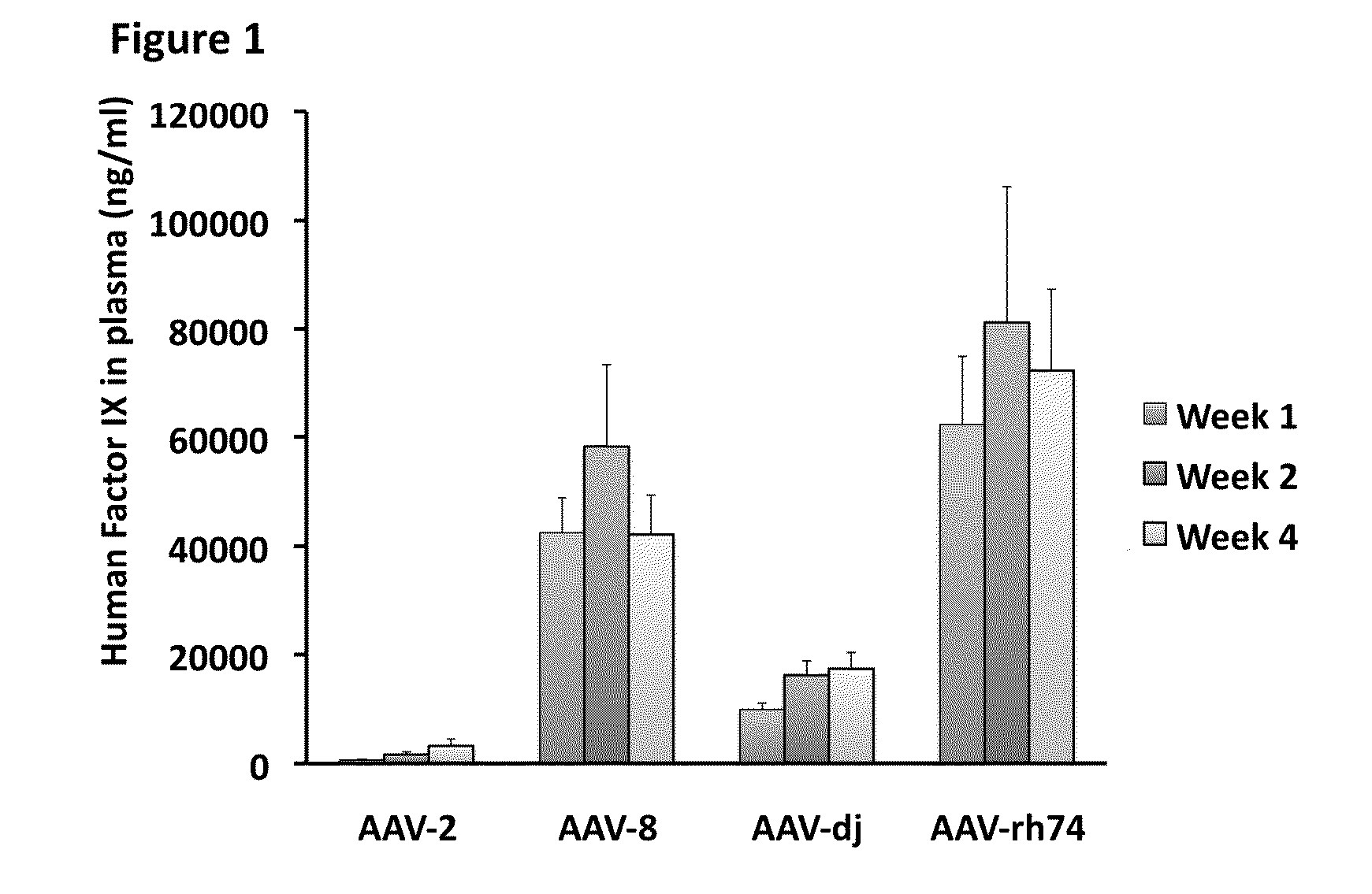
[0121]C57BL / 6 mice (n=5 per group) were injected via the tail vein with AAV vectors bearing the Factor IX (FIX) gene (2.510 vector genomes per mouse) under the control of a liver-specific promoter. Human FIX transgene product (protein) plasma levels in the mice were determined by ELISA at week 1, 2, and 4 post gene transfer, and are illustrated in FIG. 1. AAV-Rh74 showed the highest level of transgene expression in the animals.
example 3
[0122]This example includes a description of animal studies and data demonstrating effective AAV-Rh74 mediated delivery of FIX at therapeutic levels in hemophilia dogs.
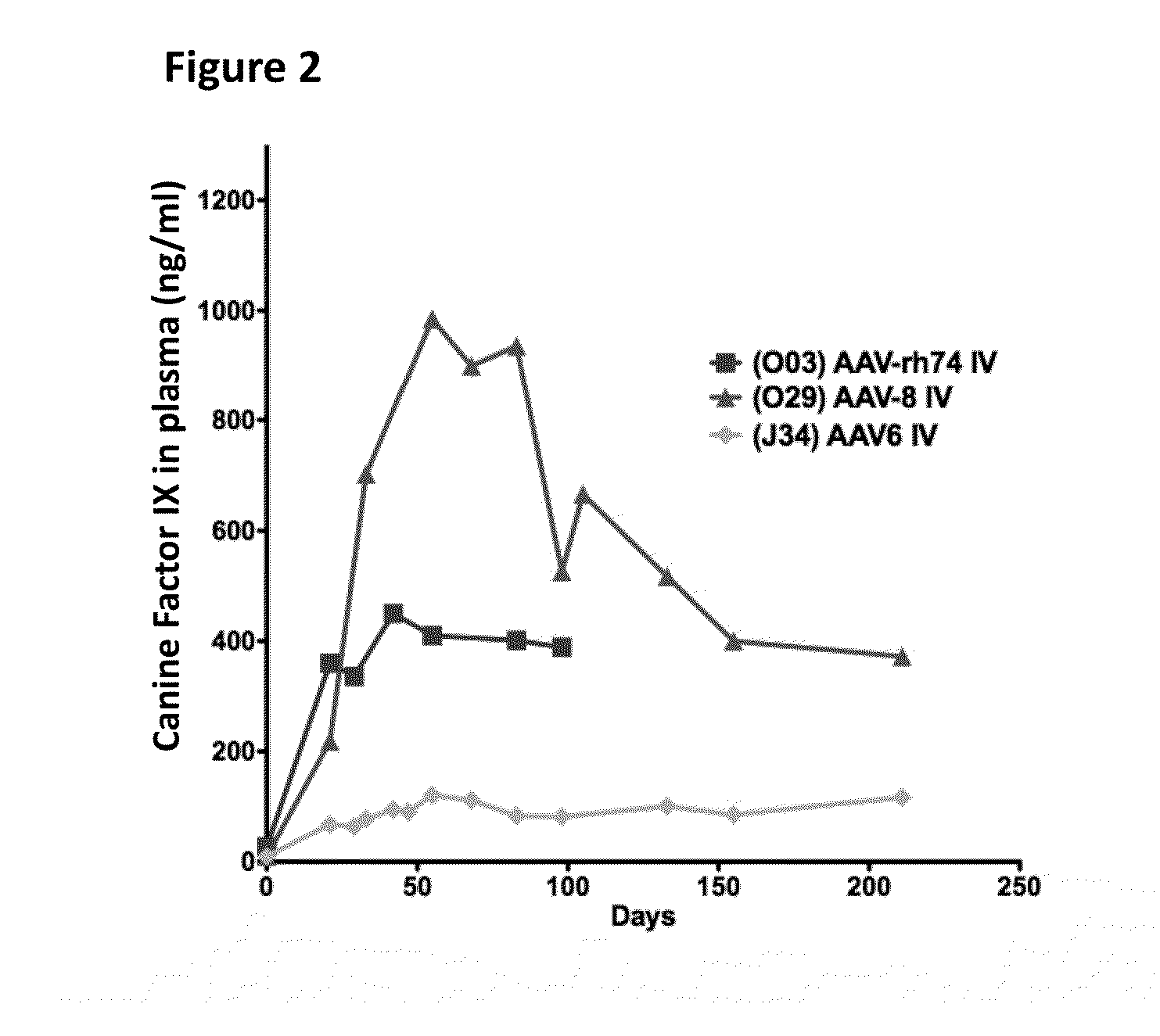
[0123]In brief, hemophilia B dogs were infused intravenously (IV) though the saphenous vein with 3×1012 vector genomes per kg of weight. Expression of the therapeutic FIX transgene was driven by a liver specific promoter. Vectors and FIX levels were monitored by ELISA. Canine FIX plasma levels are shown in FIG. 2. AAV-Rh74 and AAV8 performed roughly equally in hemophilia B dogs, and both were superior to AAV6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Atomic weight | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
| Inhibition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com