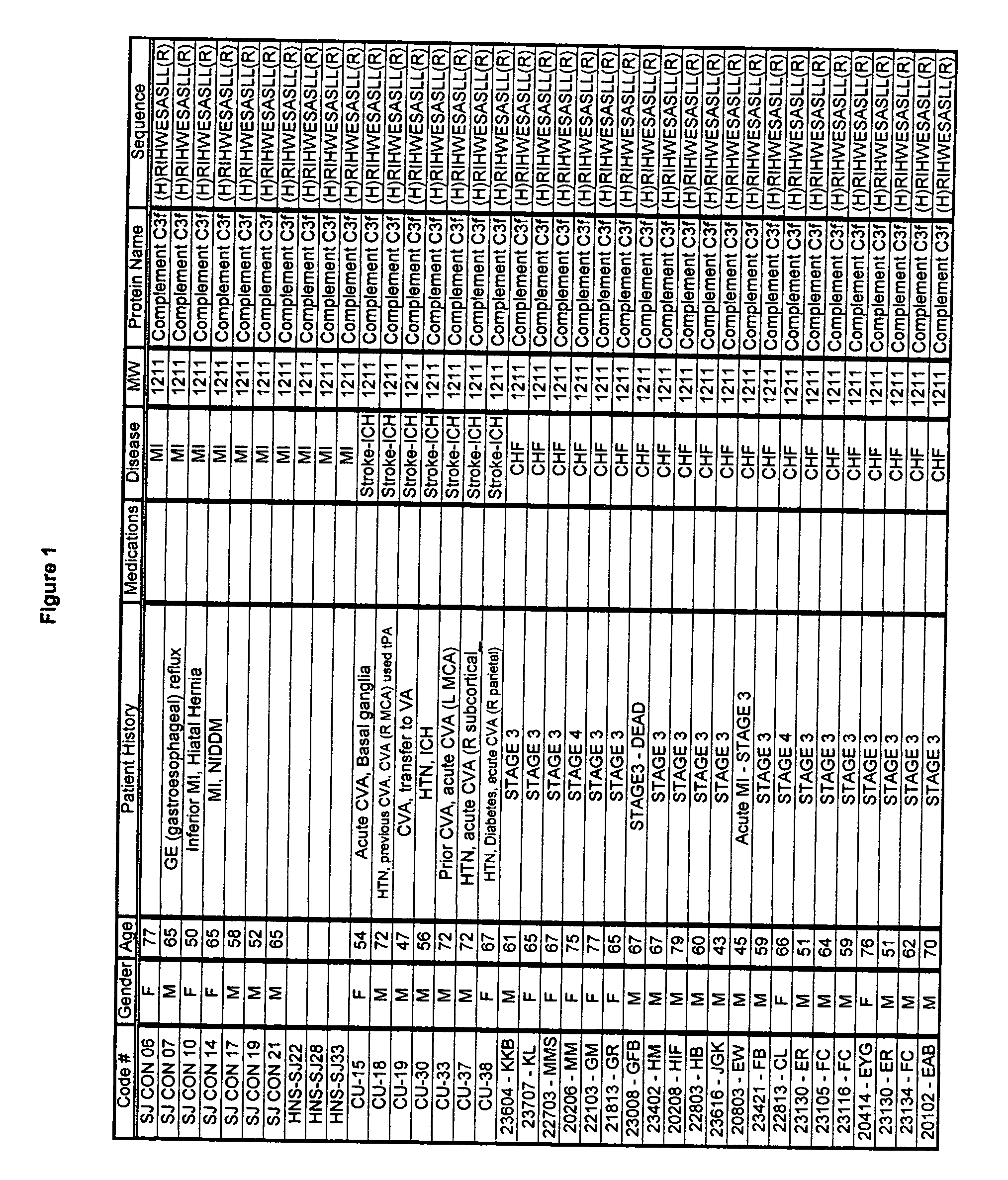
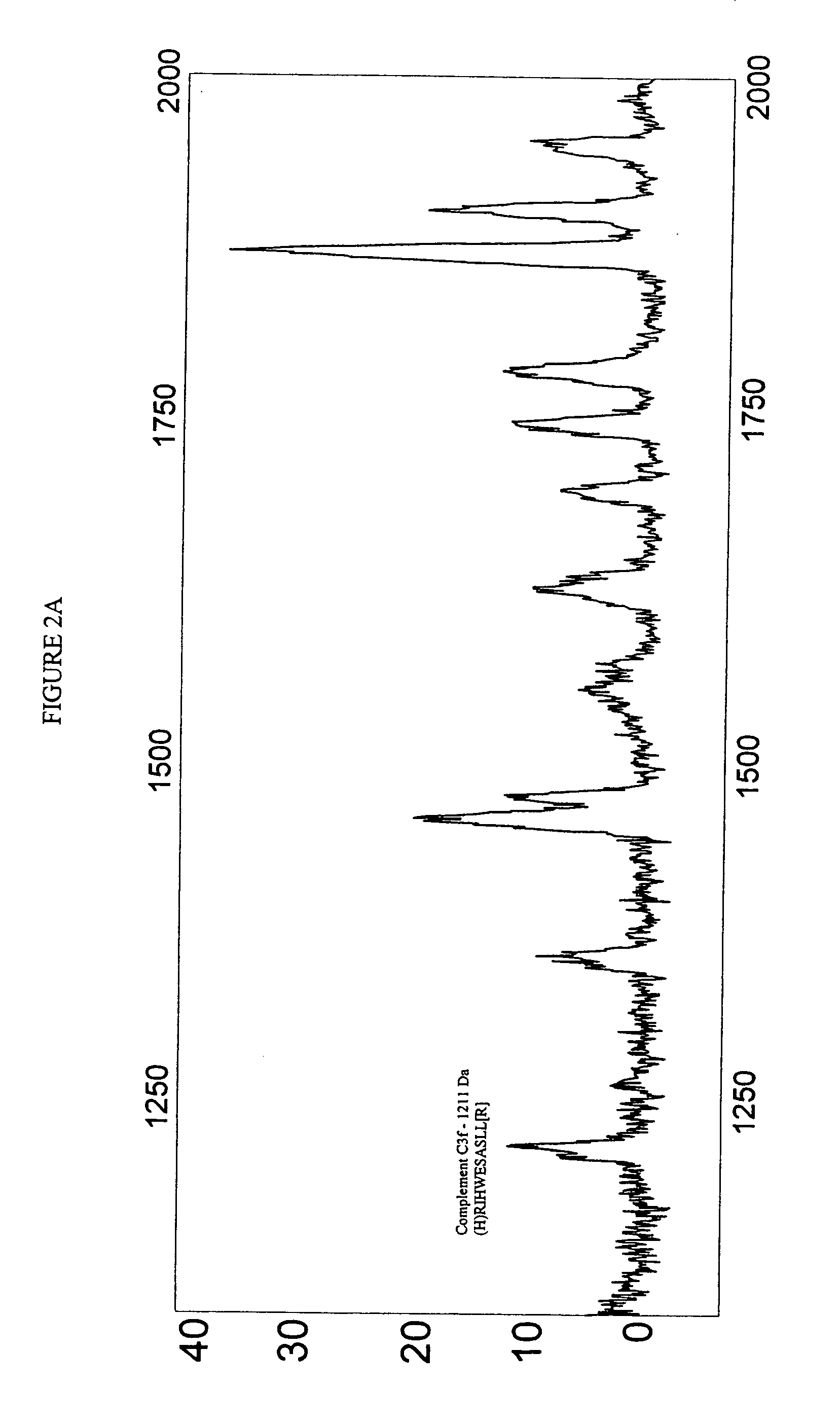
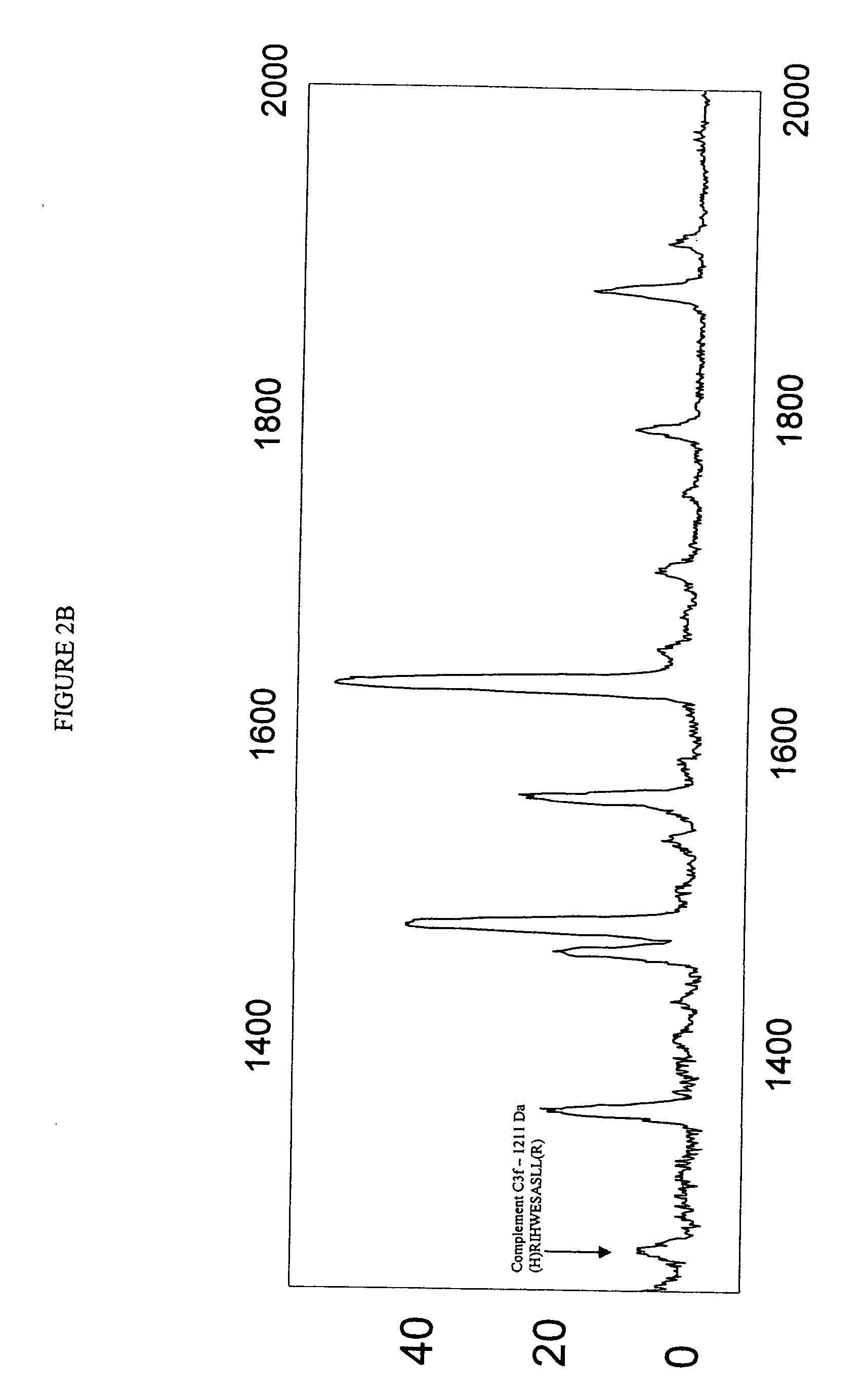
Biopolymer marker indicative of disease state having a molecular weight of 1211 daltons
a biopolymer and molecular weight technology, applied in the field of disease states, can solve the problems of not working, eam when used as free chemicals to embed analyte molecules, complicated, etc., and achieve the effect of maximizing the diversity of biopolymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] Serum samples from individuals were analyzed using Surface Enhanced Laser Desorption Ionization (SELDI) using the Ciphergen PROTEINCHIP system. The chip surfaces included, but were not limited to IMAC-3-Ni, SAX2 surface chemistries, gold chips, and the like.
[0054] Preparatory to the conduction of the SELDI MS procedure, various preparatory steps were carried out in order to maximize the diversity of discernible moities educable from the sample. Utilizing a type of micro-chromatographic column called a C18- ZIPTIP available from the Millipore company, the following preparatory steps were conducted. [0055] 1. Dilute sera in sample buffer; [0056] 2. Aspirate and dispense ZIP TIP in 50% Acetonitrile; [0057] 3. Aspirate and dispense ZIP TIP in Equilibration; solution; [0058] 4. Aspirate and Dispense in serum sample; [0059] 5. Aspirate and Dispense ZIP TIP in Wash solution; [0060] 6. Aspirate and Dispense ZIP TIP in Elution Solution.
[0061] Illustrative of the various buffering co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com