Spinal implant
a technology of artificial fusion and spinal implants, which is applied in the field of artificial fusion implants, can solve the problems of significant infection risk, less bone use, damage to nerves, etc., and achieve the effects of less blood loss, enhanced safety, and greater simplicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
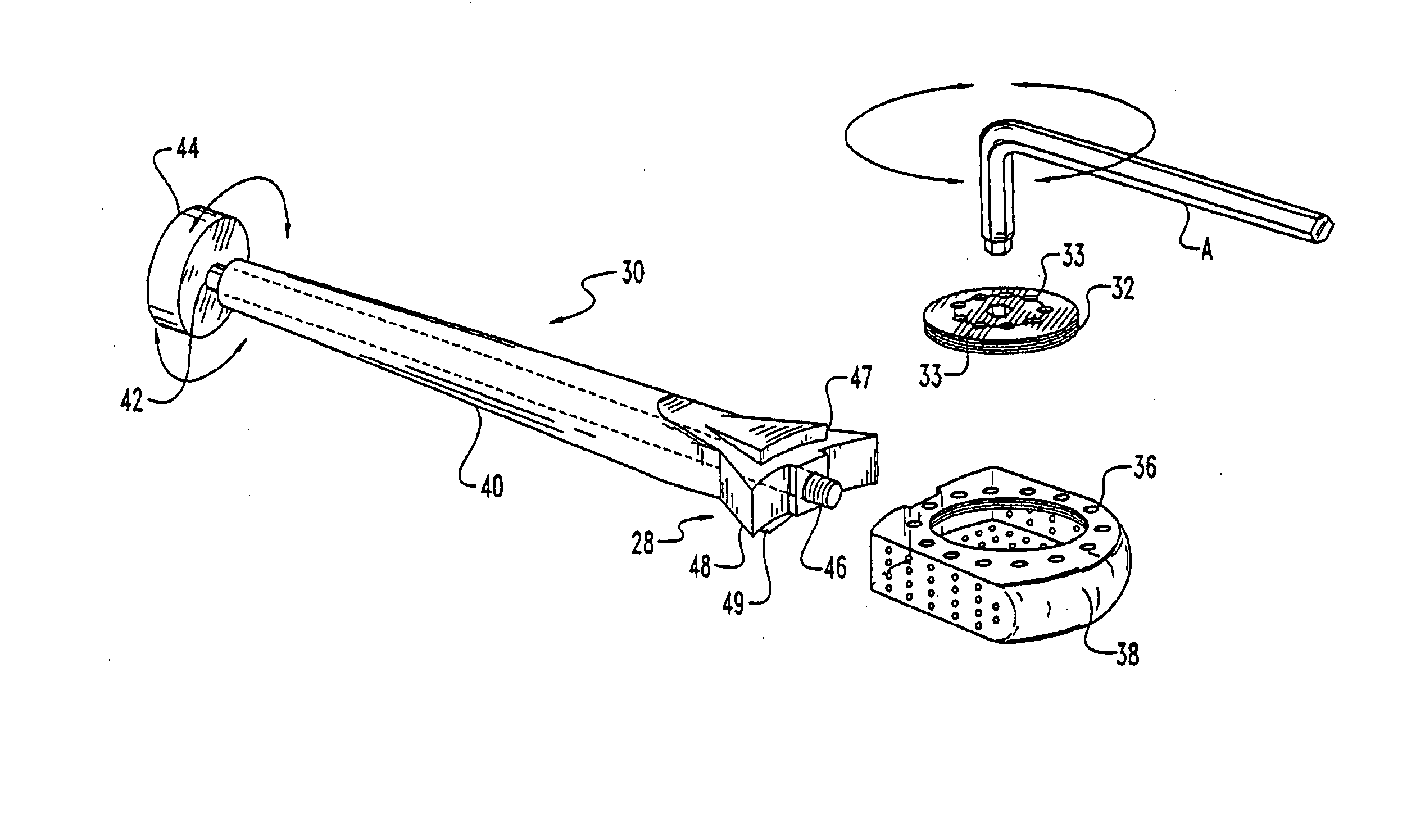
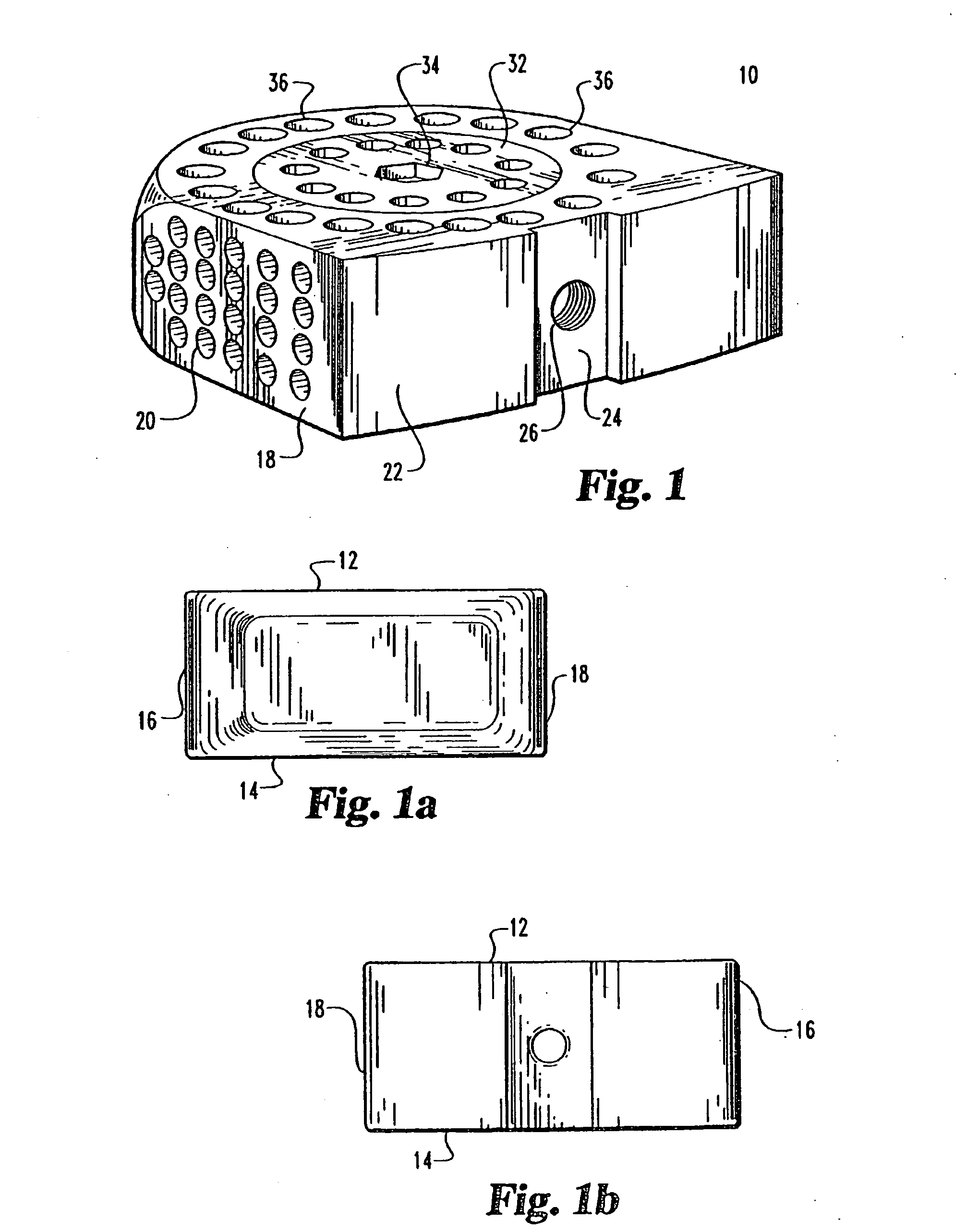
[0082] Referring to FIGS. 1 through 5 an implant for use in the disc space and associated apparatus used for inserting the implant 10 is shown. The implant 10 is shown as a substantially rectangular hollow configuration, having a tapered forward portion.
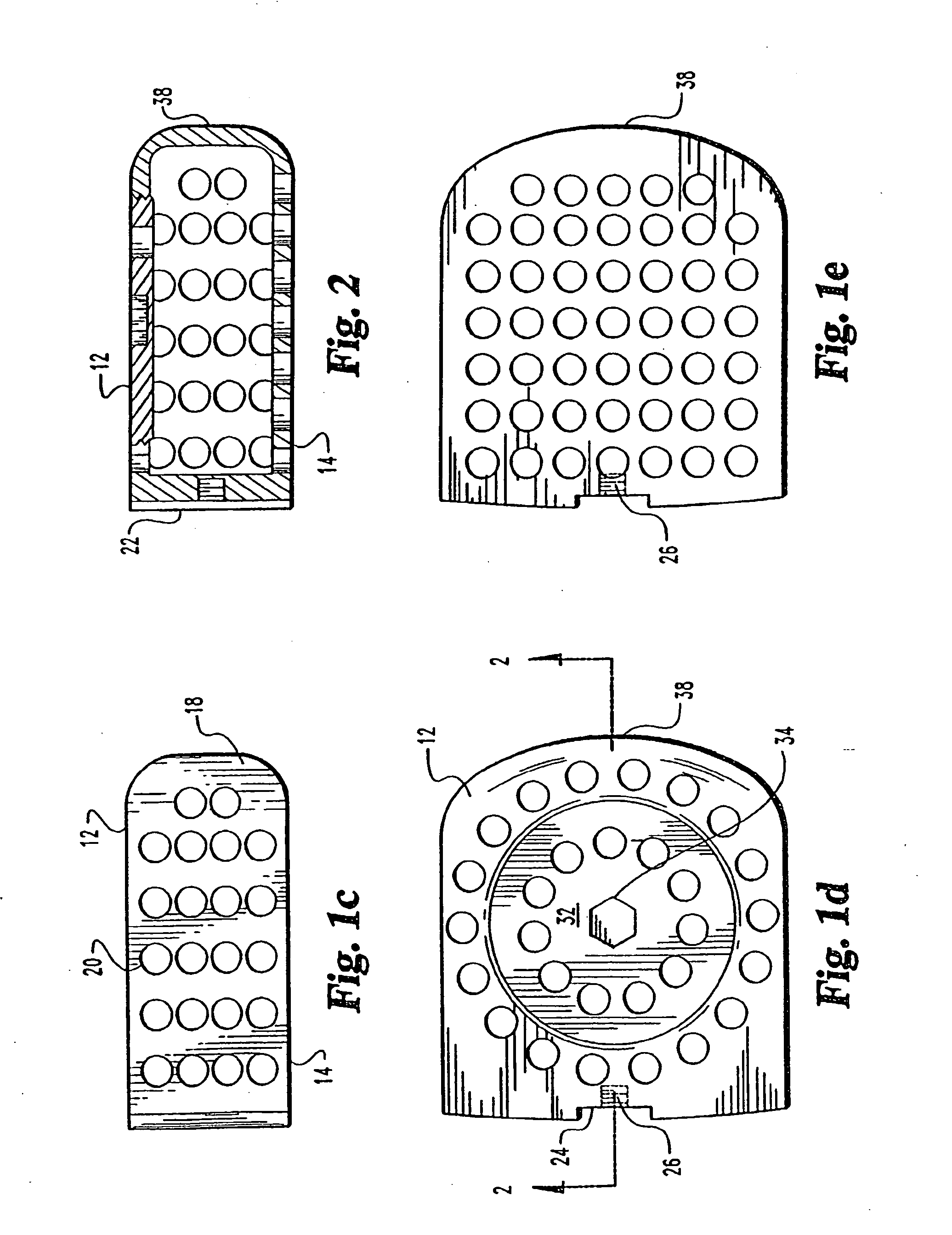
[0083] The implant 10 has an upper surface 12 and a parallel lower surface 14. The two side walls 16 and 18 are parallel to one another and have a series of small sized openings 20 of 1 mm 3 mm through the side walls 16 and 18.
[0084] The front wall 22 is slightly convex and has a depressed portion 24 with a central threaded opening 26 for receiving the engaging end 28 of a driving member 30.
[0085] The upper surface 12 has a threaded cap 32, which has opening 33 there through, with a central wrench opening 34 for engagement with an ALLEN hex key wrench A of FIG. 3. The cap 32 covers the opening into the hollow implant 10 and permits the insertion of autogenous bone material into the hollow portion of the implant 10. The cap 32 is s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com